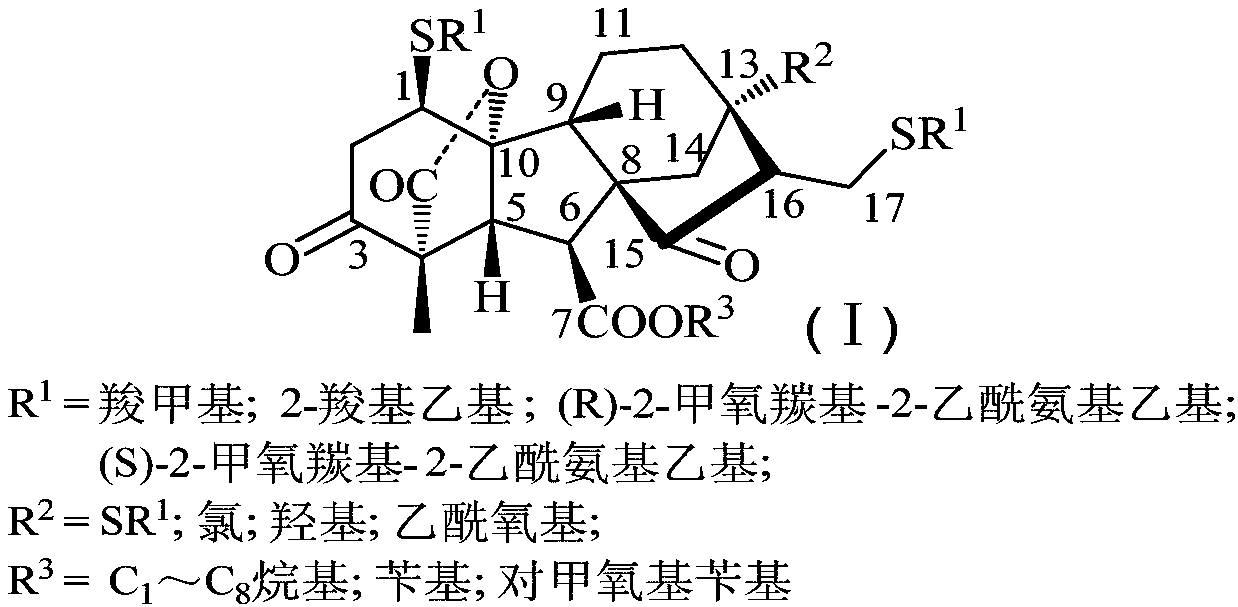
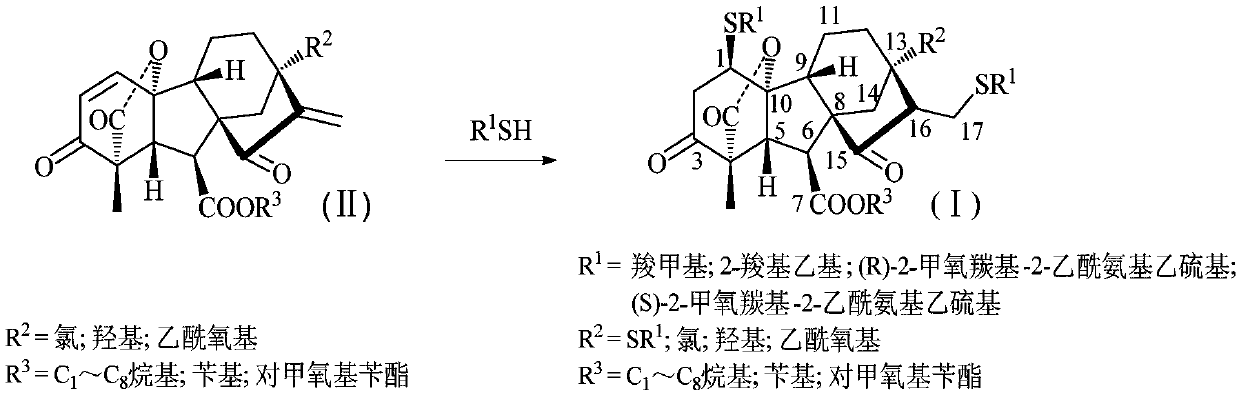
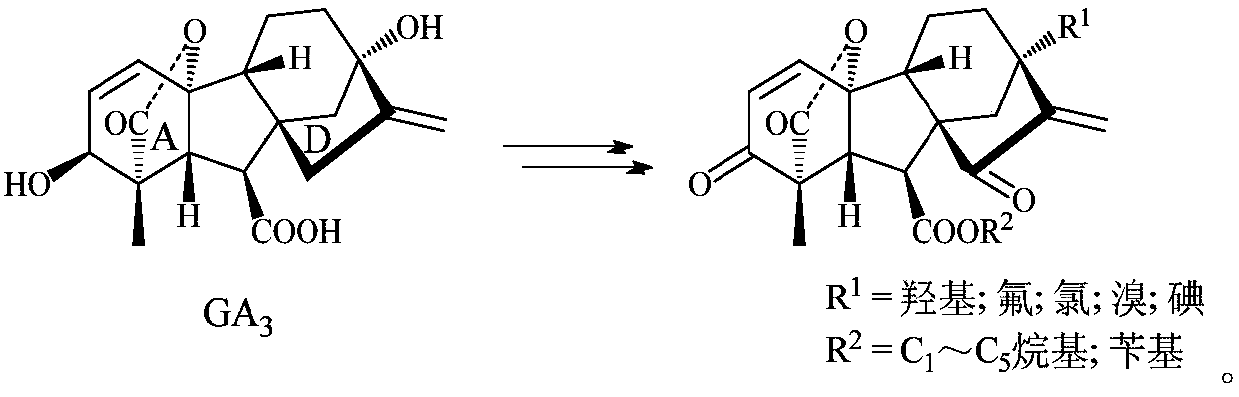
Multi-sulfo gibberellic acid ester compound, as well as preparation method and anti-tumor application thereof
A technology for polythiogibberellic acid and ester compounds, which is applied in the fields of chemistry and medicine, and can solve problems such as toxic side effects and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation and structural data of 13‐hydroxy‐3,15‐dioxo‐1,17‐dicarboxymethylthiomethyl gibberellic acid (DSG0101):
[0055]
[0056]13‐Hydroxy‐3,15‐dioxogibberellic acid methyl ester (372mg, 1.0mmol) was dissolved in chloroform (10mL), then thioglycolic acid (0.15mL, 1.33g / mL, 2.2mmol, 2.2eq. ) and diisopropylethylamine (0.050mL, 0.78g / mL, 0.3 mmol, 0.3eq.), heated at reflux at 70°C overnight. After the completion of the reaction was detected by TLC, the solvent was evaporated to dryness under reduced pressure. The residue was subjected to silica gel column chromatography (petroleum ether / ethyl acetate / acetic acid=2:3:0.005) to obtain 367 mg of white solid, yield: 66%.
[0057] 1 H‐NMR (300MHz, CD 3 OD): δ=3.79(1H,d,J=6.9Hz),3.61(3H,s),3.44(1H,d,J=9.6Hz), 3.35(2H,s),3.30(1H,q), 3.21(1H,d,J=9.6Hz), 3.00‐2.78(5H,m), 2.63(1H,t), 2.46(1H,d,J=11.4Hz), 2.12(1H,d,J=11.4Hz ),2.02‐1.95(1H,m),1.80(1H,q),1.14(3H,s);
[0058] 13 C‐NMR (75MHz, CD 3 OD): Δ = 216.62,200.00...
Embodiment 2
[0061] Preparation and structural data of 13‐hydroxy‐3,15‐dioxo‐1,17‐bis(2‐carboxyethylthio)gibberellic acid methyl ester (DSG0102):
[0062]
[0063] The preparation method is the same as in Example 1, the amount of mercaptopropionic acid is (0.19mL, 1.22g / mL, 2.2mmol, 2.2eq.), the amount and operation process of other reactants, catalysts, solvents are the same as in Example 1, and the silica gel column layer Analysis (petroleum ether / ethyl acetate=2:3) gave 467 mg of white solid, yield: 79.8%.
[0064] 1 H‐NMR (300MHz, CD 3 OD): δ=3.65(1H,d,J=6.6Hz),3.53(3H,s),3.17(1H,d,J=9.6Hz), 2.89(1H,d,J=9.6Hz),2.89‐ 2.70(5H,m),2.67(1H,d,J=9.0Hz),2.61‐2.49(7H,m),2.03(2H,d,J=11.7Hz),1.98‐1.65(4H,m),1.06 (3H,s);
[0065] 13 C‐NMR (75MHz, CD 3 OD): Δ = 216.87,200.11,175.84,174.97,172.64,96.76.70,65.41, 63.25,62.14,55.03,52.52,45.07, 42.23,35.48.7, 29.65,29.7,29.7,29.7,29.7,29.7,29.7,29.7. ,10.72;
[0066] HRMS(ESI):m / z[M‐H] ‐ calcd for C 26 h 32 o 11 S 2 :583.1313; found: 5...
Embodiment 3
[0068] Preparation and structural data of 13‐hydroxy‐3,15‐dioxo‐1,17‐bis((R)‐2‐methoxycarbonyl‐2‐acetylamino)ethylthiogibberellic acid methyl ester (DSG0103):
[0069]
[0070] 13‐Hydroxy‐3,15‐dioxogibberellic acid methyl ester (372mg, 1.0mmol) was dissolved in dichloromethane (10mL), and then N‐acetyl‐L‐cysteine methyl ester (390mg, 2.2 mmol, 2.2eq.) and triethylamine (0.138mL, 0.73g / mL, 1.0mmol, 1.0eq.), reacted overnight at room temperature, after the reaction was detected by TLC, the solvent was evaporated to dryness under reduced pressure. The residue was subjected to silica gel column chromatography (petroleum ether / ethyl acetate=2:3) to obtain 656 mg of white solid, yield: 90.3%.
[0071] 1 H‐NMR (300MHz, CDCl 3 ): δ=6.66‐6.59(2H,m),4.86‐4.79(1H,m),4.75‐4.69(1H,m),3.76(3H,s),3.74(3H,s),3.60(3H,s ),3.50(1H,d,J=7.0Hz),3.50(1H,d,J=7.0Hz),3.32(1H,d,J=9.6Hz),3.17(2H,dd,J=13.6,4.3Hz ),3.12‐3.03(1H,m),3.03‐2.97(2H,m),2.93(1H,t), 2.90‐2.83(2H,m),2.79(2H,t),2.72(1H,t), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com