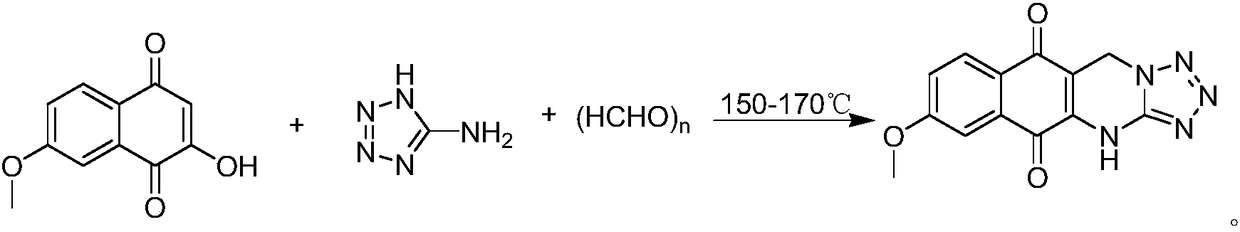1,4-naphthoquinone derivative as well as synthetic method and medical application thereof
A synthetic method and derivative technology, applied in drug combination, antineoplastic drugs, organic chemistry, etc., to achieve the effect of easy-to-obtain raw materials, simple operation, and strong anti-cancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
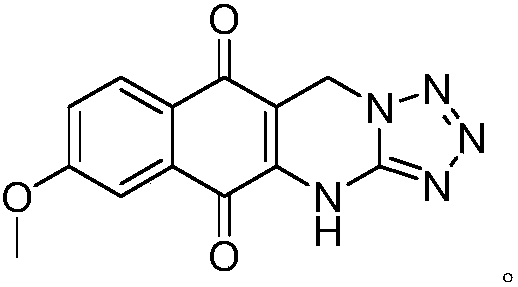
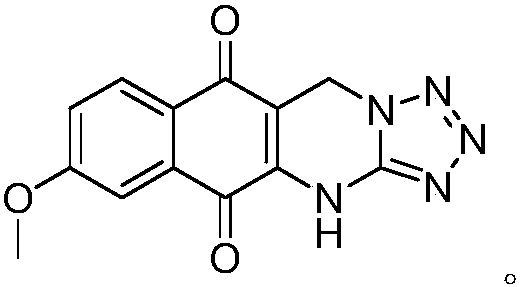
[0017] Put 0.40g of 7-methoxy-2-hydroxy-1,4-naphthoquinone, 0.17g of 5-aminotetrazole and 0.60g of paraformaldehyde in a thick-walled pressure-resistant bottle and mix evenly, the temperature is controlled at 150°C After sealing the tube and heating for 7 hours, the reaction mixture was dissolved with 20 mL of ethyl acetate, washed twice with 20 mL of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and purified by column chromatography to obtain bright yellow solid 9-methoxy-1, 0.15 g of 5-hydro-benzo[g]tetrazol[5,1-b]quinazoline-6,11-dione, the yield was 26.5%.
[0018] After testing, molecular formula: C 13 h 9 N 5 o 3 , Molecular weight: 283.07, Appearance: bright yellow solid. Melting point: >300°C. 1 HNMR (400MHz, DMSO-d 6 )δ: 11.71(s, 1H), 7.97(d, 1H, J=8.8Hz), 7.49(d, 1H, J=2.8Hz), 7.41(dd, 1H, J=2.4, 8.4Hz), 5.37( s,2H),3.95(s,3H); 13 C NMR (100MHz, DMSO-d 6 )δ: 180.8, 178.6, 163.8, 149.6, 139.1, 132.5, 128.6, 125.2, 120.9, 111.3,...
Embodiment 2
[0020] Put 0.80g of 7-methoxy-2-hydroxy-1,4-naphthoquinone, 0.34g of 5-aminotetrazole and 1.44g of paraformaldehyde in a thick-walled pressure-resistant bottle and mix evenly, the temperature is controlled at 160°C After sealing the tube and heating for 6 hours, the reaction mixture was dissolved in 40 mL of ethyl acetate, washed twice with 40 mL of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and purified by column chromatography to obtain bright yellow solid 9-methoxy-1, 0.32 g of 5-hydro-benzo[g]tetrazol[5,1-b]quinazoline-6,11-dione, the yield was 28.1%.
Embodiment 3
[0022] Put 4.0g of 7-methoxy-2-hydroxy-1,4-naphthoquinone, 1.7g of 5-aminotetrazole and 9.0g of paraformaldehyde in a thick-walled pressure-resistant bottle and mix evenly, the temperature is controlled at 170°C After sealing the tube and heating for 5 hours, the reaction mixture was dissolved in 200 mL of ethyl acetate, washed twice with 200 mL of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and purified by column chromatography to obtain bright yellow solid 9-methoxy-1, 5-Hydro-benzo[g]tetrazol[5,1-b]quinazoline-6,11-dione 1.70 g, yield 30.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com