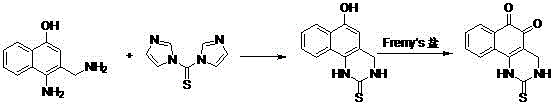
Beta-lapachone derivative, and preparation method and medicinal application thereof
A technology of derivatives and compounds, applied in the field of β-lapachone derivatives, to achieve the effect of novel structure, good selectivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthesis of embodiment 1 compound
[0021] Under the protection of argon, the compound 4-amino-3-(aminoethyl)naphthalene-1-ol (940mg, 5.0mmol) was dissolved in 20mL of dichloromethane, and the solution was cooled to 0°C with an ice bath. A solution of thiocarbonyldiimidazole (978 mg, 5.5 mmol) dissolved in 20 mL of dichloromethane was added dropwise within 10 minutes. After the addition was completed, the temperature was raised to room temperature and reacted for 5 hours. After the reaction was completed, the solvent was evaporated and washed with a small amount of ethanol to obtain the corresponding pale yellow solid. Add 20 mL of acetone to dissolve, and cool the solution to 0°C with an ice bath. Dissolve in 200mL0.06MNaH 2 PO 4 3.34 g of Fermi salt was added dropwise to the reaction solution, and the temperature was controlled below 10°C to react for 2 hours, filtered, and washed with water to obtain 538 mg of orange-yellow red solid I, with a yield of 32%. ...
Embodiment 2
[0023] Embodiment 2 in vitro antitumor activity test
[0024] The antitumor activity of the target compounds was tested by MTT method. Human liver cancer cell HepG2 was used as the test cell line, and the adherent tumor cells in the logarithmic growth phase were selected. After digesting with trypsin, 5000 cells / mL cell suspension was formulated with RPMI1640 medium containing 10% calf serum, and inoculated Inoculate 200 μL per well in a 96-well culture plate at 37°C, 5% CO 2 Incubate for 24 hours. Set up a negative control group, a positive control group and an administration group. The experimental group was replaced with new media containing different concentrations of tested samples, the control group was replaced with media containing an equal volume of solvent, and the positive control group was given the positive control drug doxorubicin (diluted with complete media to a concentration of 10 μmol L -1 ), set 3-5 parallel wells for each group, 37°C, 5% CO 2 Culture fo...
Embodiment 3
[0025] Embodiment 3NQO1 activity test
[0026] 1mL reaction system contains 25mM Tris / HCl (pH7.4), 0.7mg / mL bovine serum albumin, 0.1% Tween-20, 200μM NADH, 77μM Cytochromec, 2μg recombinant human NQOl and 1,2,3,4-tetrahydro-2- Thiobenzo[h]quinazoline-5,6-dione (25 μM). Set the detection wavelength to 550nm, add NADH to start the reaction at room temperature, calculate the reduction rate from the initial linear part of the reaction curve, and convert the molar absorption coefficient of cytochrome c (21.1mM -1 cm -1 ), the results are expressed as μmol reduced cytochrome c / min / μgNQOl. The ability of the compound to generate active oxygen can be judged from the change of the amount of cytochrome c. The rate of the compound to generate active oxygen at the enzyme level is 1143±62μmol reduced cytochrome c / min / μgNQOl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com