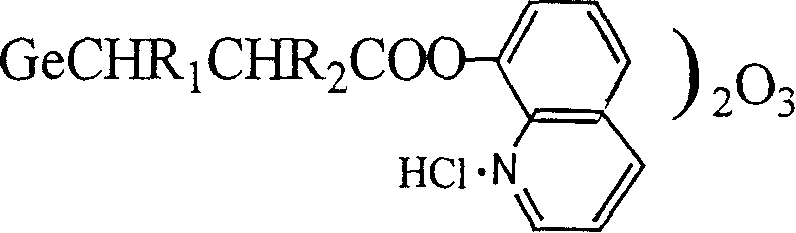Organic germanium-base quinolinate compound and its synthesis method
A synthesis method, organic germanium technology, applied in the direction of germanium organic compounds, etc., can solve the problems of high selective recognition, no anti-cancer activity, no attention to the correlation of organic groups, etc., to achieve good selective recognition and strong anti-cancer active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: synthetic quinoline ester ethyl germanium sesquioxide
[0017]
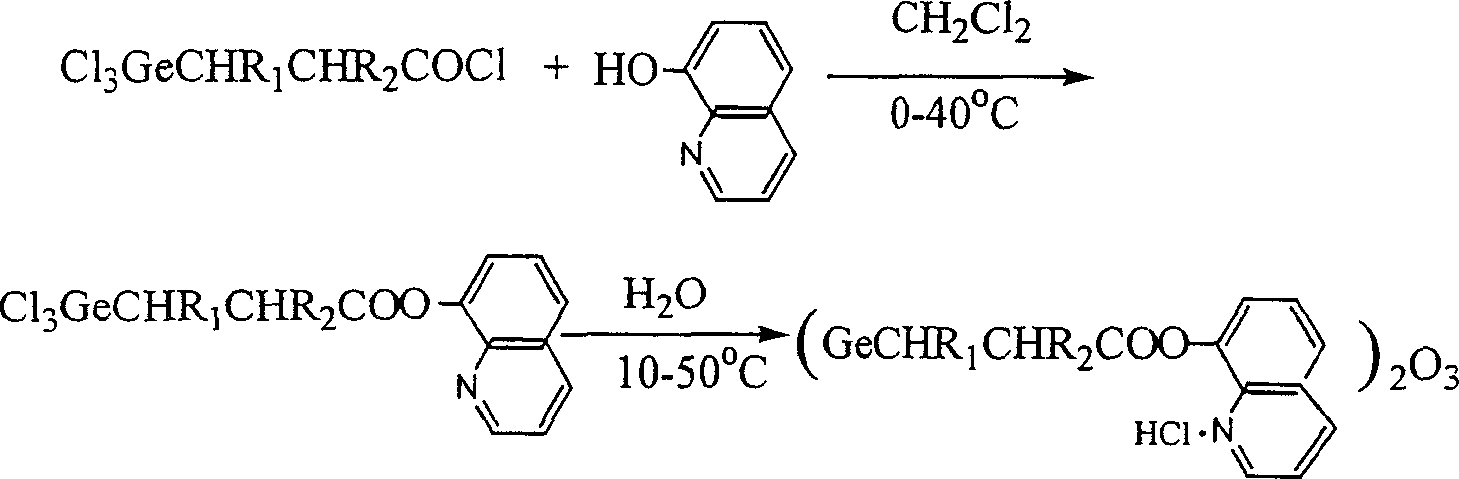
[0018] First, trichlorogermanyl propionyl chloride was synthesized according to the literature method.
[0019] Under cooling in an ice bath, 20 ml of a dichloromethane solution containing 0.025 mol of germanyl trichloropropionyl chloride was added dropwise to a solution of 40 ml of dichloromethane containing 0.05 mol of 8-hydroxyquinoline, stirred and reacted for 5 hours, and the solvent was removed by rotary evaporation, and the remaining Add 20ml of tetrahydrofuran solvent to the mixture and wash it. After the lotion is frozen, a yellow solid is precipitated, which is collected and fully washed with cold tetrahydrofuran solvent.
[0020] The solid was dissolved in 5ml of water, and after 3 minutes, the aqueous solution was transferred to 50ml of acetone at 0-5°C, stirred for 10 minutes, the precipitate was collected by filtration, rinsed with cold water, ethanol and cold acetone in turn...
Embodiment 2
[0022] Embodiment 2: synthetic quinoline ester α-methyl ethyl germanium sesquioxide
[0023]
[0024] Use α-methacrylic acid instead of acrylic acid to synthesize trichlorogermanyl-α-methacrylic acid chloride.
[0025] The experimental method and conditions are the same as those in Example 1, except that germanium trichloride α-methyl propionyl chloride is used instead of germanyl trichloride propionyl chloride, the hydrolyzate is transferred to acetone, and a light yellow viscous solid begins to appear, which solidifies after continuing to stir . The resulting product contains 1 crystal water. Yield 30%.
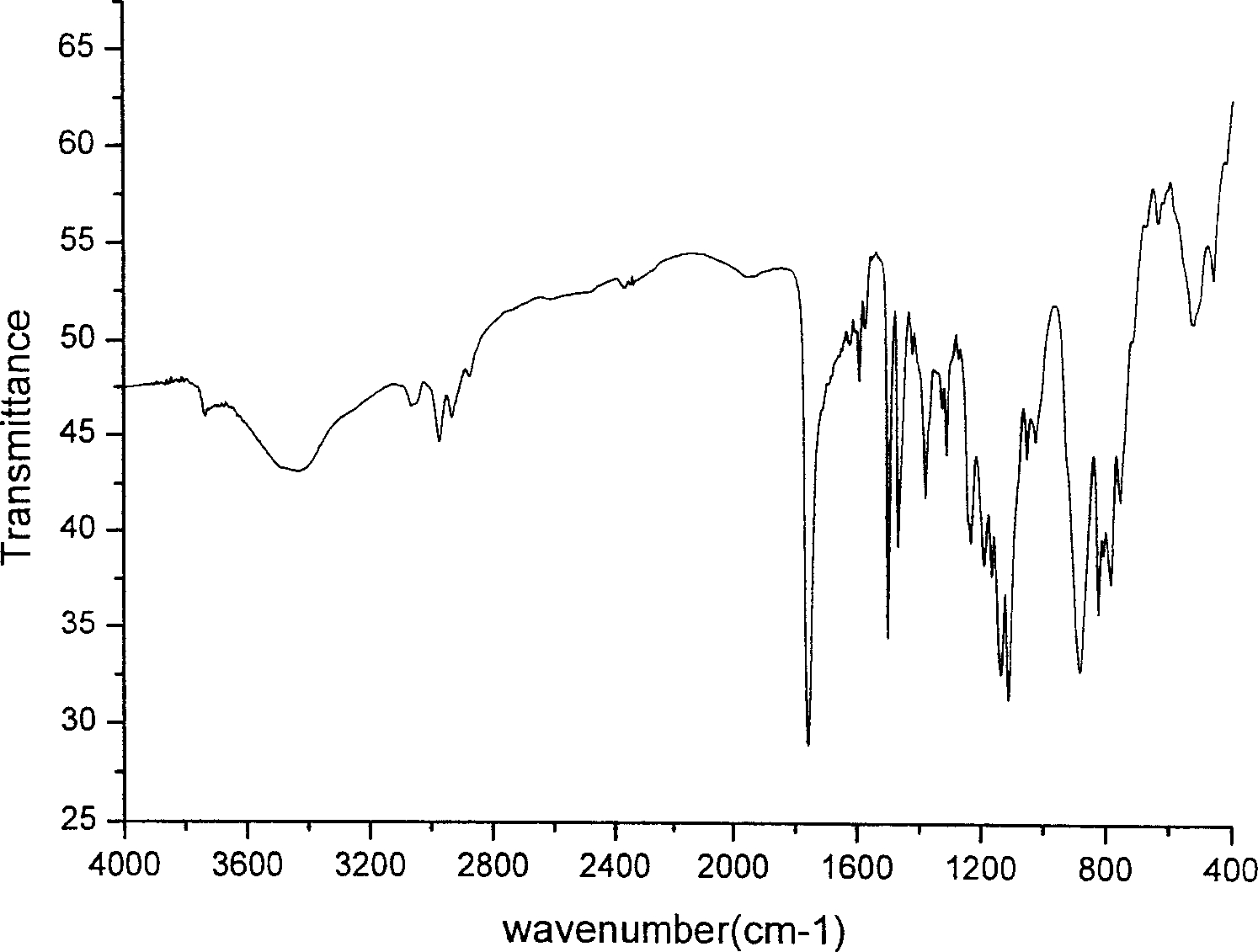
[0026] Structural characterization of the product: (1) Infrared spectrum: as shown in the attached figure, 1760cm -1 Vibration absorption peaks with strong ester bonds appear on the left and right, 800-900cm -1 Ge-O characteristic strong vibration absorption peak appears, 528cm -1 A strong vibrational absorption peak appears in Ge-C. (2) NMR: chemical shift δ=1.53:...
Embodiment 3
[0027] Embodiment 3: synthetic quinoline ester β-methyl ethyl germanium sesquioxide
[0028]
[0029] Use β-methacrylic acid instead of acrylic acid to synthesize trichlorogermanyl-β-methacrylic acid chloride.
[0030] The experimental method and conditions are the same as those in Example 2, except that germanyl trichloride-β-methylpropionyl chloride is used instead of germanyl chloride α-methylpropionyl chloride. The resulting product contains 1 crystal water. Yield 62%.
[0031] Structural characterization of the product: (1) Infrared spectrum: 1755cm -1 There are strong ester bond vibration absorption peaks on the left and right, 883cm -1 There is a strong vibrational absorption peak characteristic of Ge-O, 525cm -1 A strong vibrational absorption peak appears in Ge-C. (2) NMR: chemical shift δ=1.51:3H, multiplet, -CH 3 ; δ=2.72:2H, triplet, -CH 2 ; δ=3.2-3.7:1H, multiplet, -CH; δ=7.2-8.0:6H, multiplet, quinoline hydrogen. (3) Elemental analysis C 26 h 26 N 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com