Application of 2-imino-phenylate-5-(2-hydroxy-benzyl)-1,3-thiazole-4-ketone in preparing anti-cerebral cancer medicaments
A technology of imino and benzyl, applied in the preparation of anti-brain cancer drugs of 2-phenyl ether imino-5-(2-hydroxy-benzyl)-1,3-thiazol-4-one to achieve the effect of strong anticancer activity and great development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of 2-phenylene ether imino-5-(2-hydroxy-benzyl)-1,3-thiazol-4-one
[0050]
[0051] According to HCl: H 2 The ratio of O (1:4) is dubbed hydrochloric acid solution. Weigh 11.4g (150mmol) of ammonium thiocyanate and dissolve it in 50ml of hydrochloric acid solution, add 10.5ml (100mmol) of 4-phenoxyaniline, and heat to 85°C under stirring conditions, the mixture becomes clear, after 12 hours of reaction, TLC monitoring After the reaction, the mixture was cooled to room temperature, and a viscous oily liquid appeared, which was extracted with ethyl acetate, and the extract was washed with 10% hydrochloric acid solution, saturated sodium chloride solution, and water in sequence, and the extract was evaporated under reduced pressure to remove the solvent. , to obtain 4-phenoxyphenylthiourea.
[0052] Add 1110mg (6.0mmol) of 4-phenoxyphenylthiourea and 2479mg (30mmol) of anhydrous sodium acetate into 20ml of ethanol, add 1.28ml (12mmol) of ethyl chloroacetat...
Embodiment 2
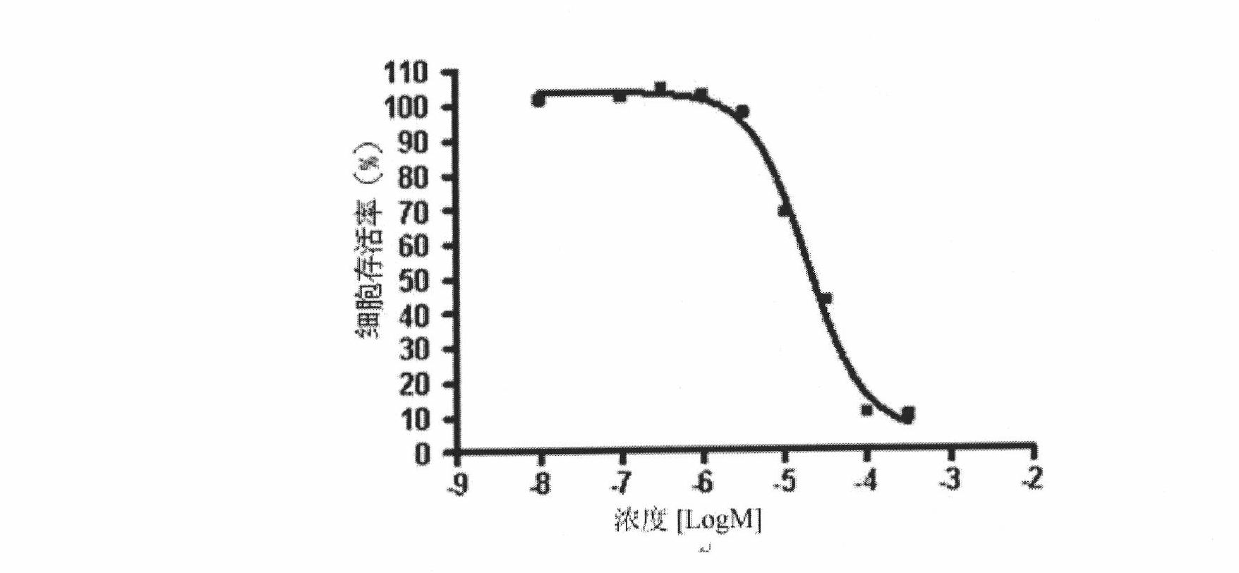
[0056] Selective Anticancer Activity Assays
[0057] Two cell lines (human brain glioma cell U87MG, HegG2 cell whose morphology is similar to normal human cells) were used to screen the compound synthesized by the present invention for anticancer activity in a conventional method.
[0058] at 37°C with 5% CO 2 Under the environment of DMEM medium, U87MG cells were cultured, and HepG2 cells were cultured in 1640 medium, and the logarithmic phase U87MG and HepG2 cells were collected and seeded in 96-well plates (2×10 4 After 24 hours of incubation, the compounds of the present invention were added at different concentrations (100, 50, 20, 10, 5, 1, 0.1, 0.01 μM). After 24 hours of action, 10 μl of WST-1 was added to each well, and then continued to incubate for 2 hours. , the cell viability was detected by enzyme-linked immunoassay to detect its absorbance, the dose-effect relationship was studied, and the 2-phenylene ether imino-5-(2-hydroxy-benzyl)-1,3- was analyzed by SigmaP...
Embodiment 3
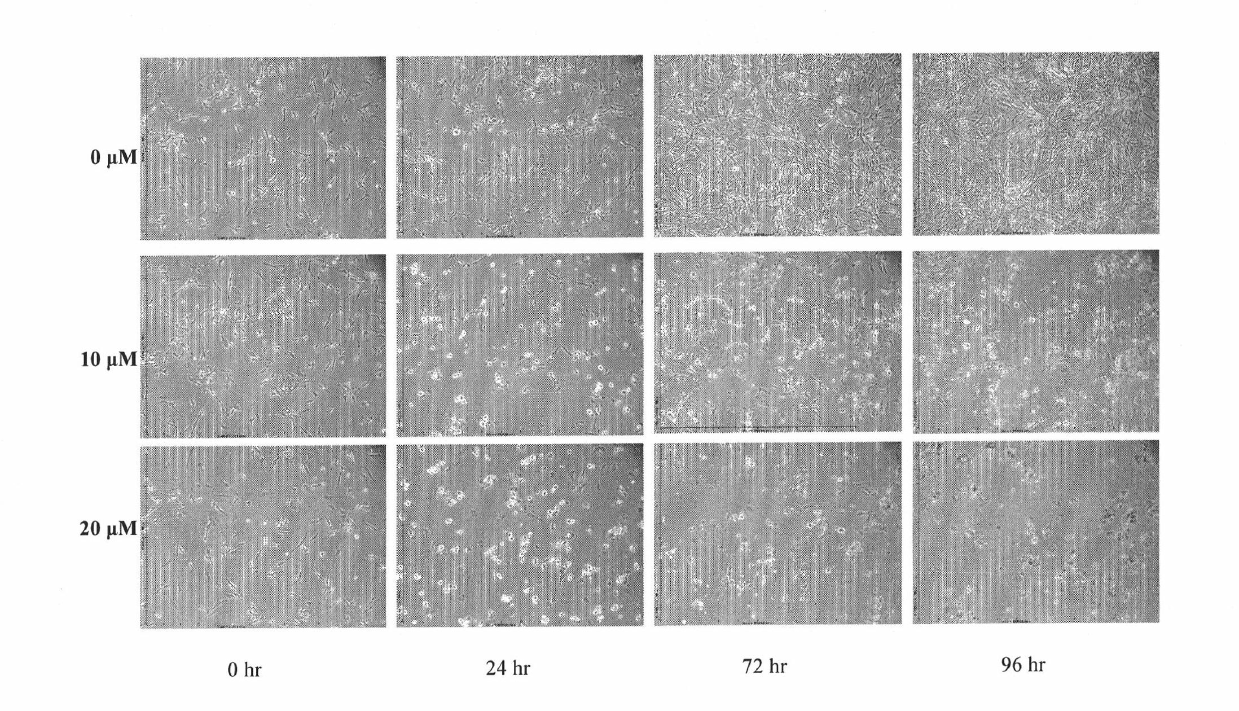
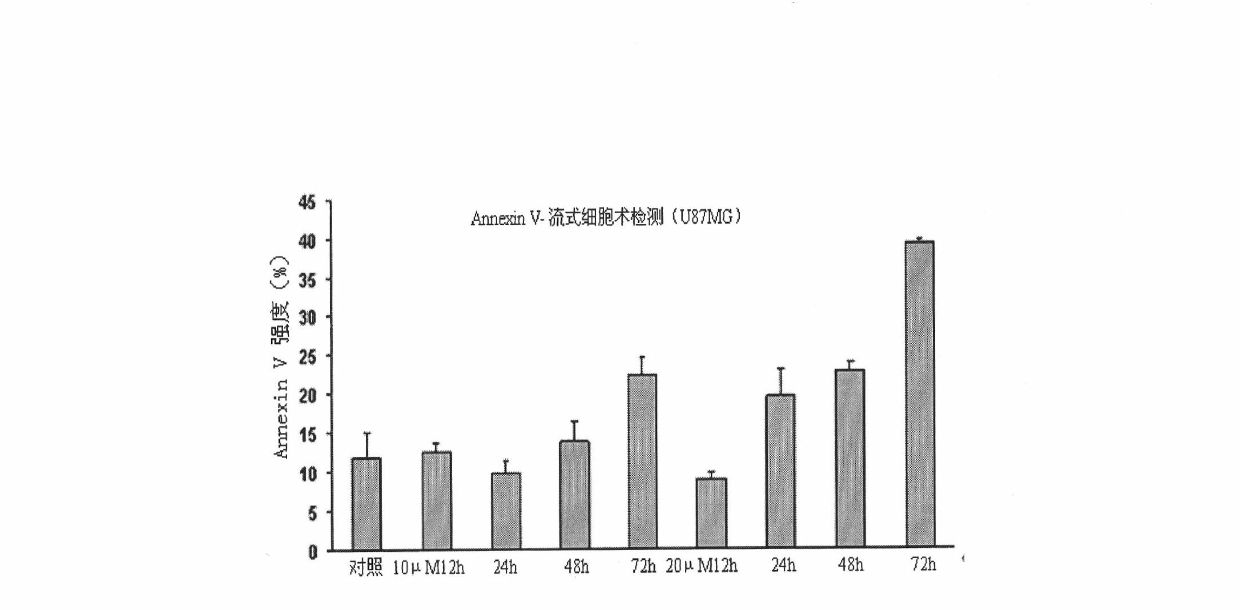
[0060] Time-effect relationship of 2-phenylene ether imino-5-(2-hydroxy-benzyl)-1,3-thiazol-4-one on cell proliferation and cell morphology
[0061] Collect U87MG and HepG2 cells in logarithmic phase, inoculate in 96-well plate, 37°C with 5% CO 2 After incubating for 24 hours under the environment of 0, 10, and 20 μM, the compound 2-phenylene ether imino-5-(2-hydroxy-benzyl)-1,3-thiazole-4- Ketone, after 24h, 72h and 96h, respectively, observe the effect of the compound on the proliferation and morphology of U87MG cells (results in figure 1 ). As a result, the longer the action time, the stronger the effect of the compound on the cells, and the effect of 2-phenylene ether imino-5-(2-hydroxyl-benzyl)-1,3-thiazol-4-ketone on U87MG has time dependence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com