Lung non-cellule cancer early stage specific self antibody panel diagnostic kit
A technology for non-small cell carcinoma and non-small cell lung cancer, applied in biological testing, material inspection products, measuring devices, etc., can solve the problem of insufficient sensitivity and specificity to meet lung cancer screening, and achieve high diagnostic value and operability Effects of Simplicity, Sensitivity, and Specificity Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preliminary screening experiment:
[0048] Serum collection: The serum of the control group came from healthy people in the physical examination center. All lung cancer cases have been diagnosed by histopathology, according to the latest WHO classification of lung cancer and the 6th UICC staging standard for lung cancer, and meet the following requirements: ①Patients with primary lung non-small cell carcinoma (including squamous cell carcinoma) confirmed by histopathology or cytology cell carcinoma (squamous cell carcinoma), adenocarcinoma, large cell carcinoma). ②Have not received any anti-cancer treatment before blood collection. ③ All patients signed the informed consent.
[0049] First, the patient's serum was hybridized with a chip containing 1627 disease-related proteins to obtain specific binding highlights. The chip was scanned by MicroVigene software (Vigene Tech version 2.9.9.2) to obtain the optical density value. The antigenic protein specifically...
Embodiment 2
[0055] Example 2 Medium screening experiment scheme:
[0056] 1) Serum collection: Serum from patients with benign lung tumors: ① No anti-cancer treatment was received before blood collection. ② All patients signed the informed consent. The inclusion criteria for all lung cancer cases were the same as the initial screening.
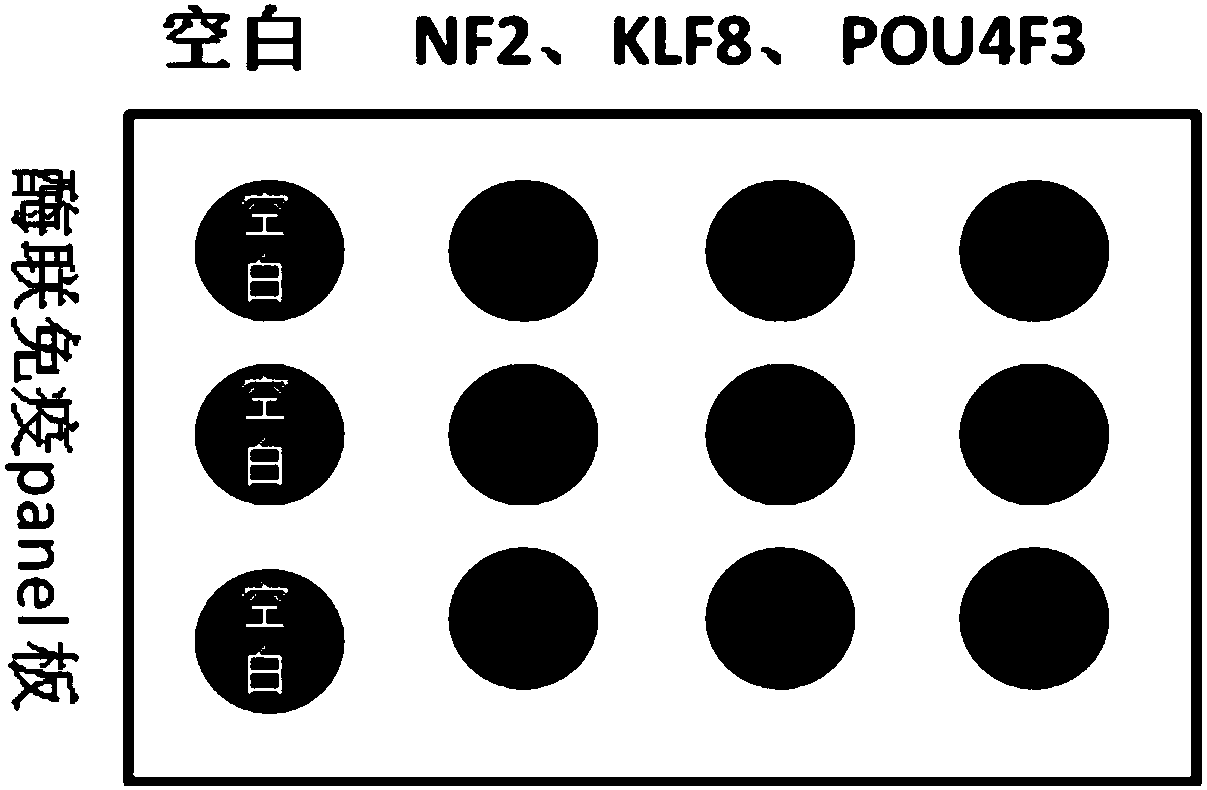
[0057] 2) Screening in the protein chip: Print the antigenic proteins corresponding to the 10 initially screened antibodies on glass slides to make chips, add patient serum or control serum to each chip, add rabbit anti-human secondary antibody, scan, Statistical analysis.
[0058] The basis for screening autoantibodies is: on the premise that the specificity is greater than 90%, the sensitivity is above 20%.
[0059] The 6 autoantibodies were HOXA1, IKZF3, KLF8, NF2, POU4F3, and TLX2 obtained through intermediate screening.
[0060] Conclusion: In the screening experiment, the above 6 autoantibodies were obtained after statistical analysis.
Embodiment 3
[0061] Embodiment 3 Verification stage experimental scheme:
[0062] 1) Serum collection: The principle of collection is the same as that of the primary screening stage. But there can be no repeat serum.
[0063] 2) Use ELISA (enzyme-linked immunosorbent assay) method to detect the relative expression level (OD value) of the effective antibody obtained after screening. The method is as follows: in a 96-well plate, after incubation overnight with the antibody coated with anti-GST, the corresponding antigenic protein with the GST label is added, and the antigen is captured by the anti-GST and coated in the well plate. Then add experimental serum to incubate, finally add rabbit anti-human secondary antibody, TMB substrate color development, OD450 reading value.
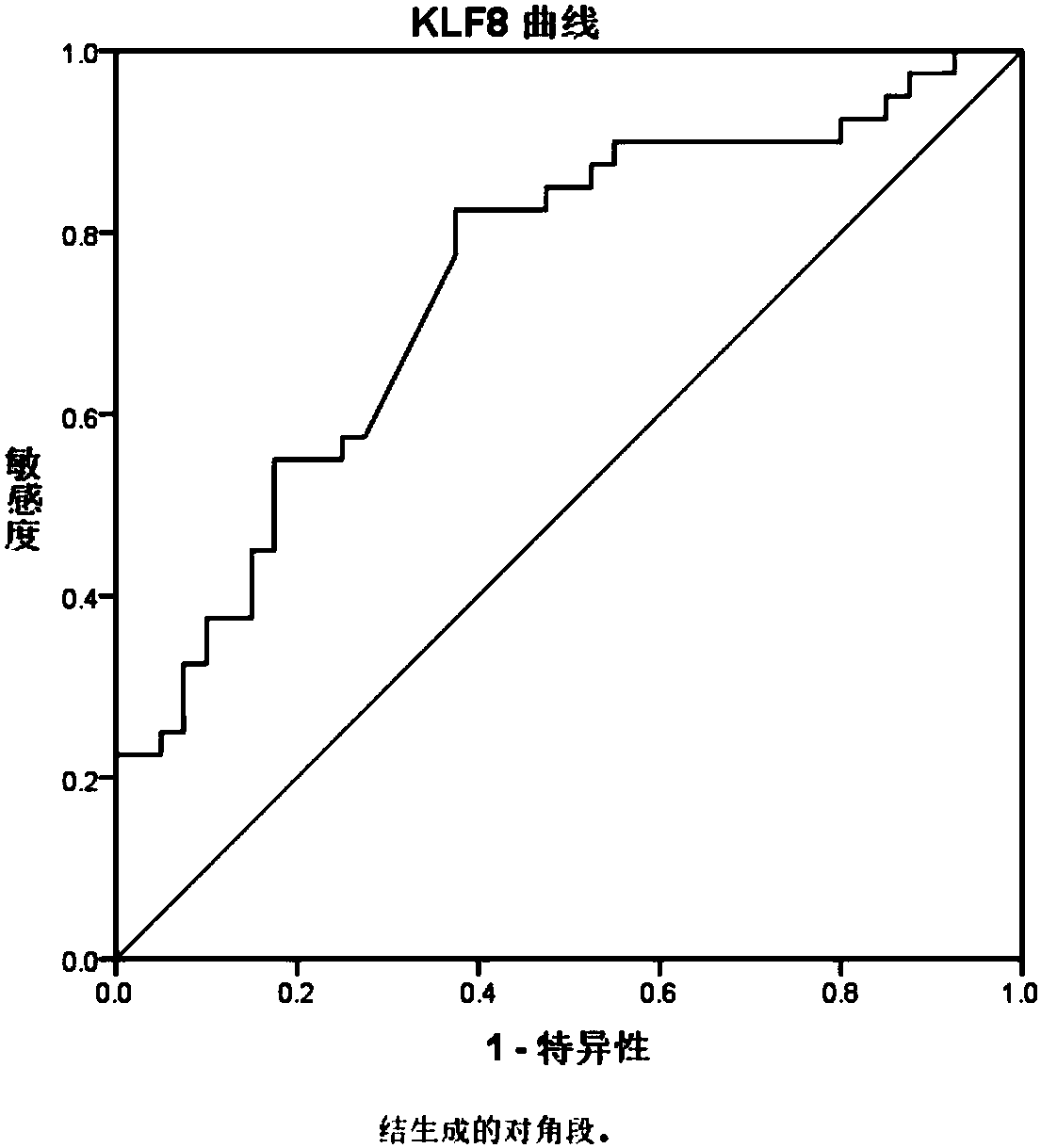
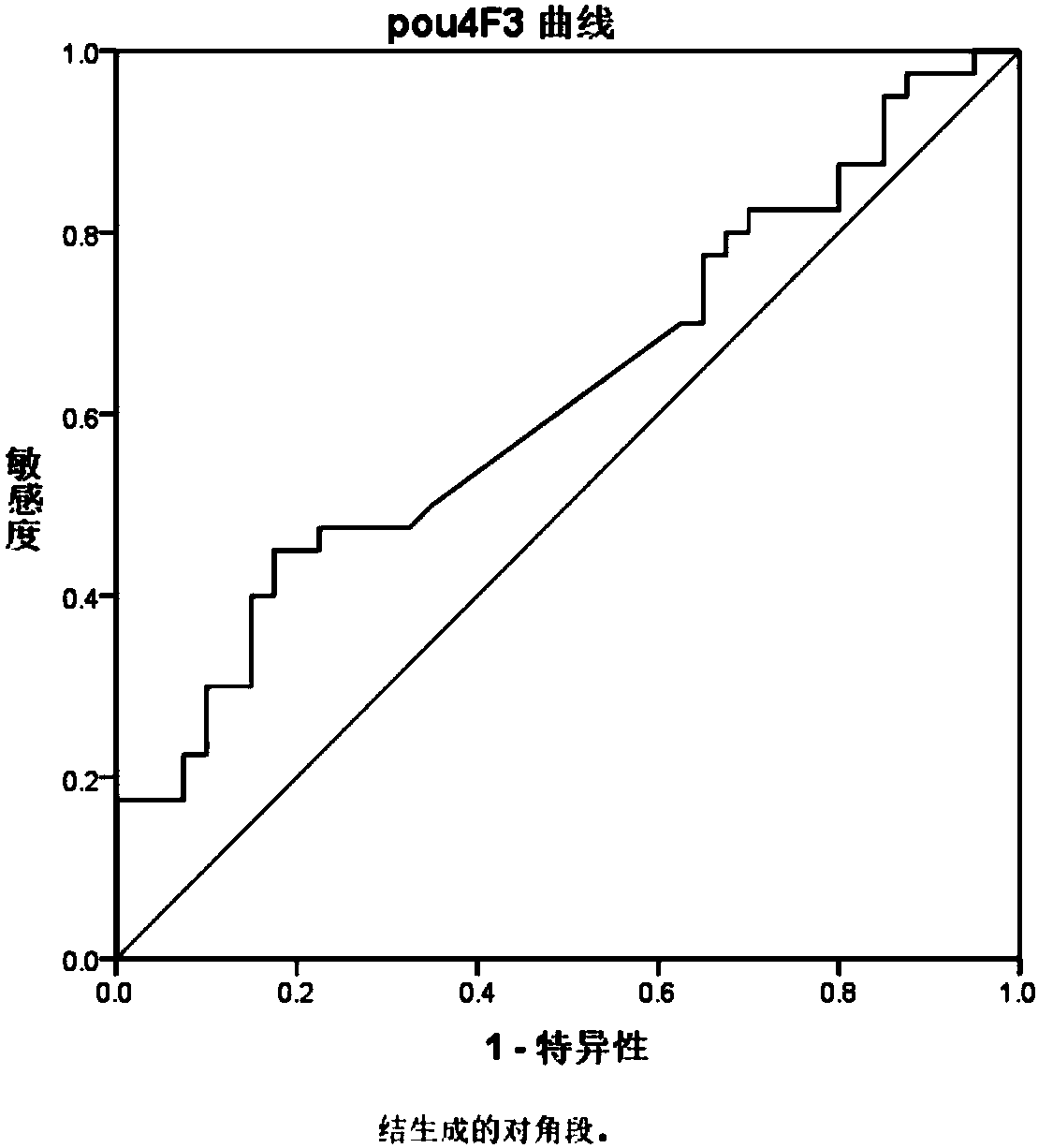
[0064] Basis for screening autoantibodies: under the premise that the specificity is greater than 90%, the sensitivity is above 30%.
[0065] Conclusion: The specific autoantibodies NF2, KLF8 and POU4F3 in three sera ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com