Composite injection for treating or preventing anesthesia hypotension and preparation method of composite injection
A technology for compound injections and injections, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve problems such as the use of methoxamine alone, and achieve low adverse reaction rates and high stability. Good, the effect of maintaining heart rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
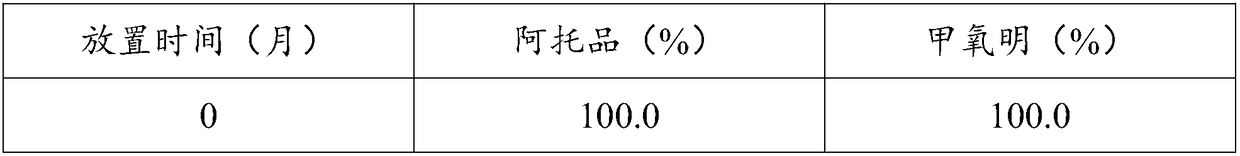
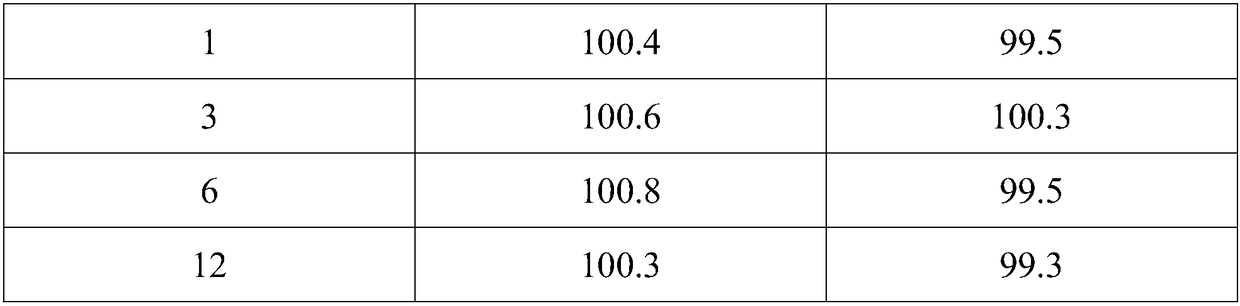
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing the compound injection described in the above technical solution, comprising the following steps:
[0037] (1) dissolving atropine sulfate and methoxamine hydrochloride with part of water to obtain an active solution;
[0038] (2) mixing the active solution with pharmaceutical excipients to obtain a mixed solution;
[0039] (3) The mixed solution is mixed with the remaining water, followed by filter sterilization and high-temperature sterilization in sequence to obtain a compound injection.
[0040]In the present invention, part of water is firstly used to dissolve atropine sulfate and methoxamine hydrochloride to obtain liquid A, wherein said part of water accounts for 1 / 2 to 2 / 3 of the total volume of water. In the present invention, the temperature of the water for dissolving atropine sulfate and methoxamine hydrochloride is 35-40°C, more preferably 37-39°C. The invention adopts warm water (35-40 DEG C) to ...
Embodiment 1
[0046] Mix 0.2 g of atropine sulfate and 2 g of methoxamine hydrochloride with 35° C. and 666 mL of water for injection, stir and dissolve sufficiently to obtain liquid A. Mix 6 g of citric acid, 3 g of sodium citrate, and 3.5 g of sodium chloride with liquid A, stir and dissolve thoroughly to obtain liquid B. Dilute liquid B to 1000mL with water for injection to obtain a mixed liquid, filter it with a 0.22μM sterilizing filter, pack the filtered solution into ampoules and seal it, and sterilize it by circulating steam at 100°C for 30 minutes to obtain treatment or Compound injection for preventing anesthesia hypotension.
Embodiment 2
[0048] Mix 0.5 g of atropine sulfate and 3 g of methoxamine hydrochloride with 35° C. and 666 mL of water for injection, stir and dissolve sufficiently to obtain liquid A. Mix 6 g of citric acid, 3 g of sodium citrate, and 3.5 g of sodium chloride with liquid A, stir and dissolve thoroughly to obtain liquid B. Dilute liquid B to 1000mL with water for injection to obtain a mixed liquid, filter it with a 0.22μM sterilizing filter, pack the filtered solution into ampoules and seal it, and sterilize it by circulating steam at 100°C for 30 minutes to obtain treatment or Compound injection for preventing anesthesia hypotension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com