A kind of sirpα fusion protein and preparation method and use thereof
A fusion protein and composition technology, applied in the field of fusion proteins, can solve the problems of effectiveness, safety and production cost limitations, and achieve the effects of reducing production costs, improving phagocytosis, and enhancing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0040] Experimental Example 1 Preparation of SIRPα fusion protein of the present invention
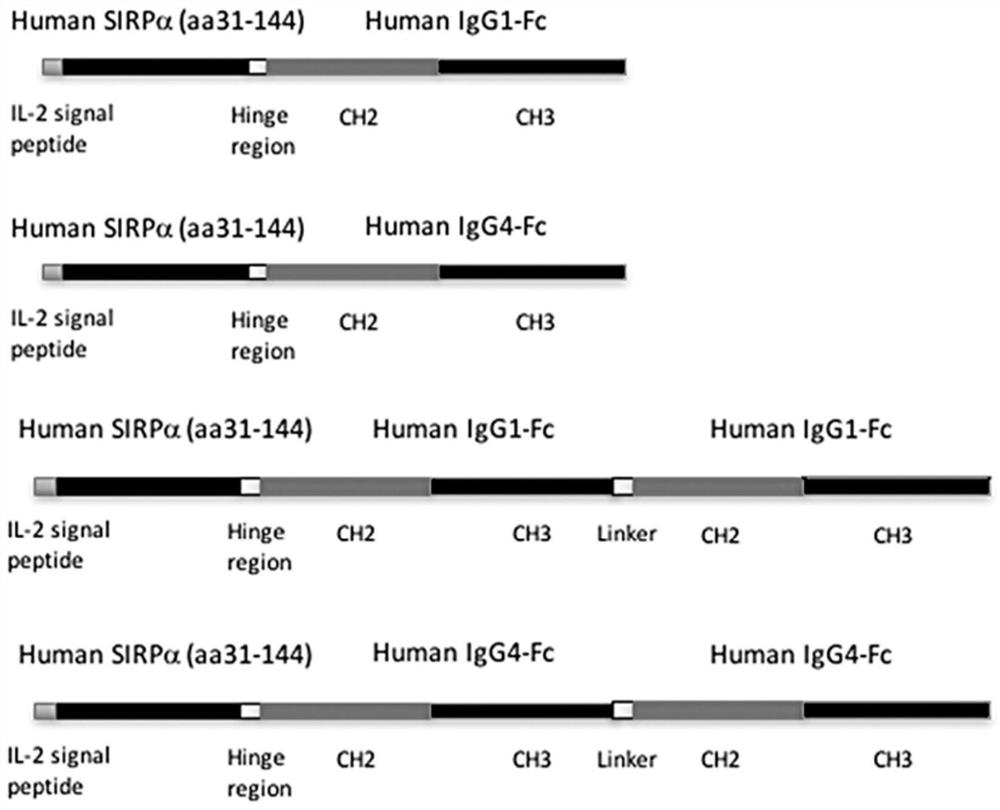
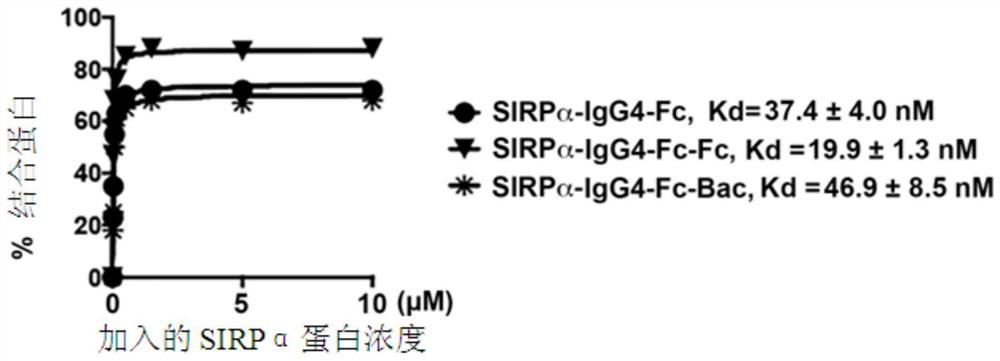
[0041] Such as figure 1 Shown is a schematic structural diagram of the SIRPα fusion protein of the present invention. SIRPα-Fc and SIRPα-Fc-Fc fusion proteins are composed of human IgG1 Fc region (SEQ ID NO. 2), human IgG4 Fc region (SEQ ID NO. 3) , human IgG1 Fc-Fc or human IgG4 Fc-Fc fused with the N-terminal V domain (variable region domain) of human SIRPα (SEQ ID NO. 1) to obtain a fusion protein.
[0042] Among them, the same human SIRPα domain is connected to the human IgG4 Fc region containing the hinge stabilizing S288P mutation that prevents the formation of disulfide bonds in the chain, and SIRPα-Fc (Fc is human IgG4 Fc) and SIRPα-Fc-Fc (Fc It is human IgG4 Fc-Fc) fusion protein, and its sequences are respectively shown in SEQ ID NO. 8 and SEQ ID NO. 9.
[0043] All fusion proteins described above were produced by overlapping PCR using standard molecular biology techniques ...
experiment example 2
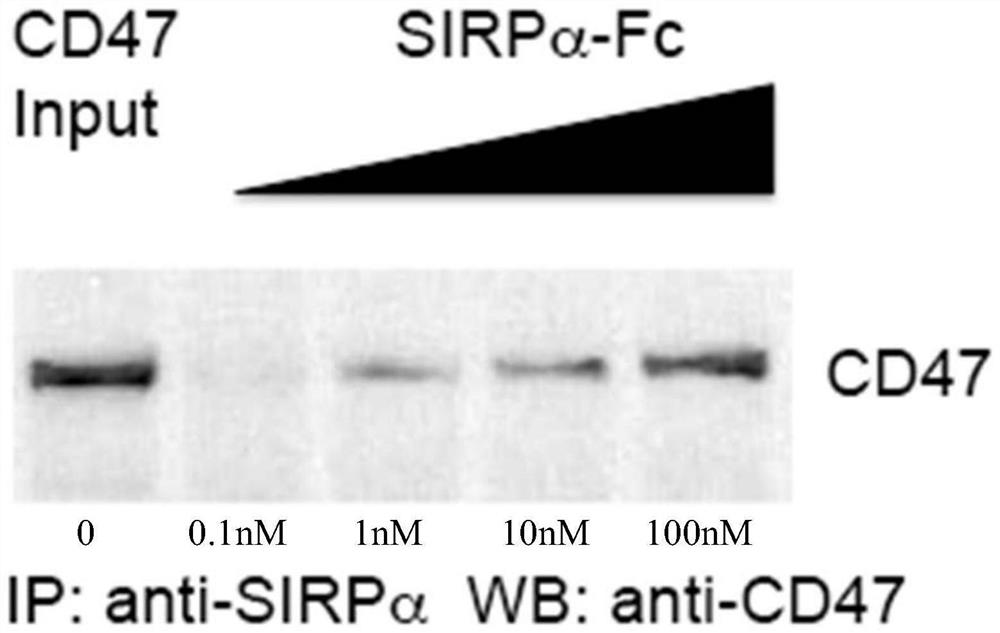
[0058] Experimental example 2 The binding effect of SIRPα-lgG4-Fc fusion protein of the present invention on CD47
[0059] (1) Western blot experiment of binding of SIRPα-lgG4-Fc fusion protein of the present invention to CD47
[0060] Mix the above purified fusion protein with different concentrations (equal ratio increase, 0.1nM, 1nM, 10nM, 100nM) with purified CD47 protein (10ng, Abcam) and protein A sepharose beads in PBS solution, 4 degrees Overnight, the immune complexes were pelleted by centrifugation and run on SDS-PAGE gel electrophoresis. Finally, CD47 protein levels were quantified by Western blot assay. Wherein, the amount of CD47 added was directly used as a control.
[0061] Such as figure 2 As shown, it is the western blotting diagram (IP, Immunoprecipitation, immunoprecipitation experiment; WB, Western Blotting, immunoblotting experiment) of the SIRPα-lgG4-Fc fusion protein of the present invention that carries out IP-WB experiment, it can be seen that with...
experiment example 3
[0067] Experimental example 3 Induction of the phagocytosis effect of the SIRPα-lgG4-Fc fusion protein of the present invention in vitro
[0068] (1) Preparation of M1 macrophages
[0069] Human M1 macrophages were prepared from monocytes obtained from normal healthy human donors (human peripheral blood mononuclear cells were provided by StemCelltechnologies). Monocytes were differentiated into macrophages by culturing in special differentiation medium (supplied by StemCell technologies) supplemented with M-CSF (Macrophage Colony-Stimulating Factor) (20 ng / mL), Specific steps are as follows:
[0070] Monocytes were differentiated into macrophages by culturing in RPMI 1640-based differentiation medium for 6-10 days. The medium was supplemented with the following components: 10% heat-inactivated human AB serum, 1% GlutaMax, 1% penicillin and streptomycin (purchased from GIBCO LifeTechnologies).
[0071] One day before the measurement of phagocytosis, IFN-γ (Interferon-γ, γ-in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com