A method for measuring the biological potency of heparin drugs
A technology of biological titer and determination method, applied in the field of biological analysis, can solve the problems of high price, low sensitivity and high test cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation of the test product includes: centrifuging the reaction solution after adding the quenching agent, and taking the supernatant as the test product.
[0039] Further, in order to facilitate the subsequent quantitative determination, the supernatant was mixed with an equal volume of internal standard to obtain the test substance to be tested.
[0040] Step S2: Determination of the content of the reaction product in the potency system by high performance liquid chromatography-mass spectrometry analysis;
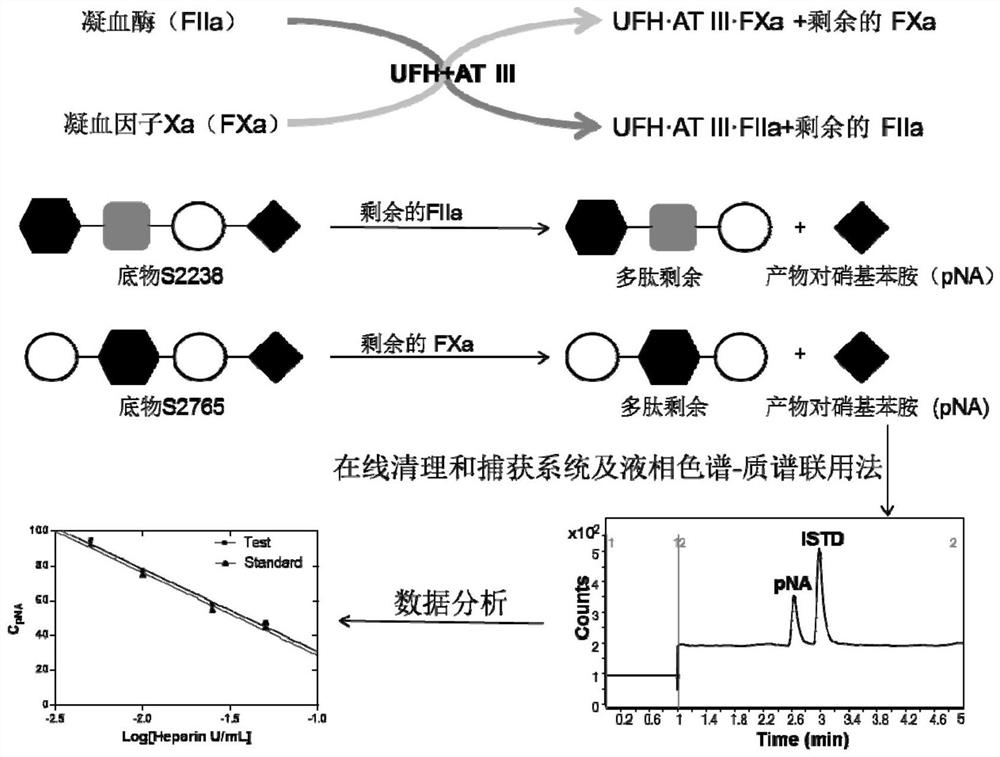
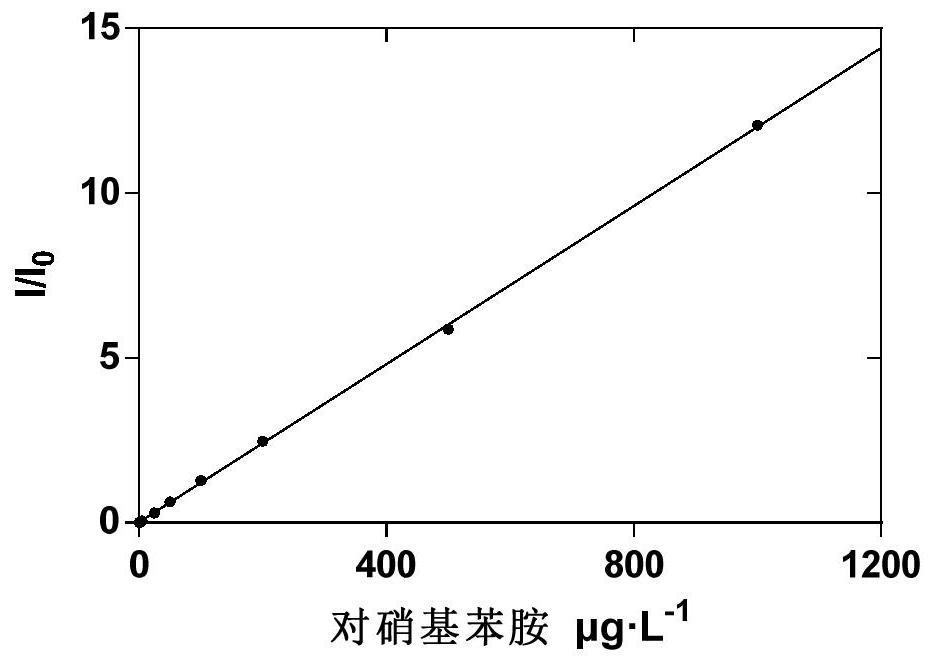
[0041] Utilize the principle that the substrate produces the corresponding products p-nitroaniline (pNA), 7-amino-4-methylcoumarin, benzoyl arginine and 4-nitrophenol under the action of the corresponding coagulation factor respectively. The products p-nitroaniline, 7-amino-4-methylcoumarin, benzoyl arginine and 4-nitrophenol were used as quantitative standards.
[0042] Further, the content of the reaction product was determined by the internal standard m...
Embodiment 1
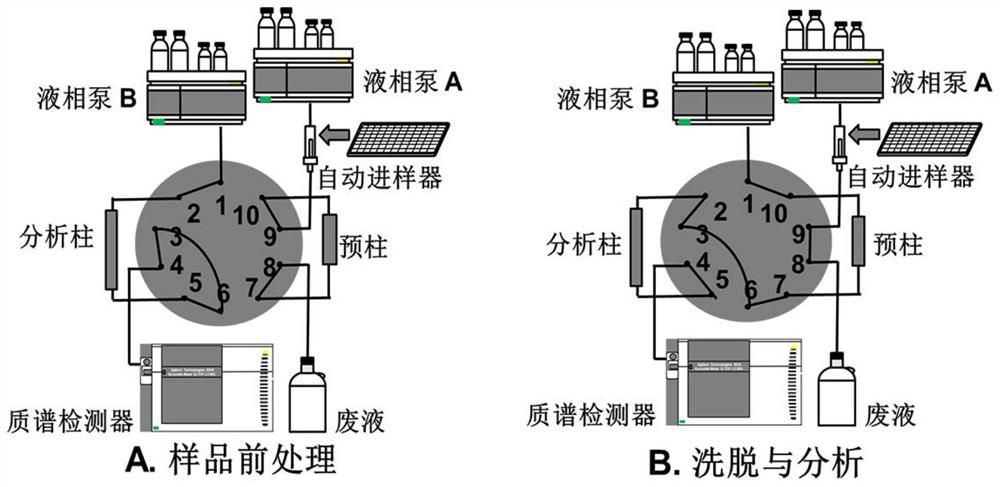
[0062] This embodiment provides a method for assaying the biological potency of heparin sodium, and its detection principle is as follows: figure 2 shown, which includes:
[0063] 1. Establish an in vitro anticoagulant factor (IIa, VIIa, IXa, Xa, XIa, XIIa) titer response system:
[0064] 1. Solution preparation:
[0065] Prepare tris-polyethylene glycol 6000 buffer (pH=8.4): 50mM Tris, 7.5mM EDTA, 175mM NaCl, 0.1% PEG 6000, add 800mL water, adjust pH=8.4 with hydrochloric acid, dilute with water to 1000mL as Buffer solution: Accurately weigh an appropriate amount of coagulation factor (IIa, IXa, Xa, XIa, XIIa, VIIa) powder, and dilute it to 0.05IU·mL with buffer solution -1 , 0.25IU·mL -1 , 0.015IU·mL -1 , 0.25IU·mL -1 , 0.5IU·mL -1 and 0.25IU·mL -1 Accurately weigh an appropriate amount of substrate powder, and use distilled water to mix S2238, D-Leu-Phg-Arg-4-nitroanilide (LPAN), S2765 and Z-Gly-Gly-Arg-7-amido-4-methylcoumarin (GGAM), Benzoyl arginineethyl ester (...
Embodiment 2~10
[0105] Drugs as shown in Table 4, fondaparinux, dalteparin, adeparinux, nadroparin, certoparin, paraparin, reviveparin, tinzaparin and enoxaparin, were used instead of heparin sodium in Example 1 , select suitable substrate according to table 5 and establish anticoagulant factor Ea (E=II, VII, IX, X, XI, XII) potency system, establish the online sample pretreatment high performance liquid chromatography-mass spectrometry of corresponding product Combined analysis method. Use the standard as a reference standard to measure the biological potency of different heparins against different coagulation factors. The result is consistent with the result of Experiment 1, which is 90-110% of the marked value, and the titer ratio of the anti-Xa factor to the anti-IIa factor meets the regulations.
[0106] Table 4. Heparin Drugs
[0107]
[0108] Table 5. Enzymes and substrates available in the anticoagulant factor Ea potency system
[0109]
[0110]
[0111]
[0112]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com