Azeotropic distillation process for separating butanol and butyl acetate
A technology of butyl acetate and azeotropic distillation, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, to achieve the effects of reducing solvent loss, reducing costs, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
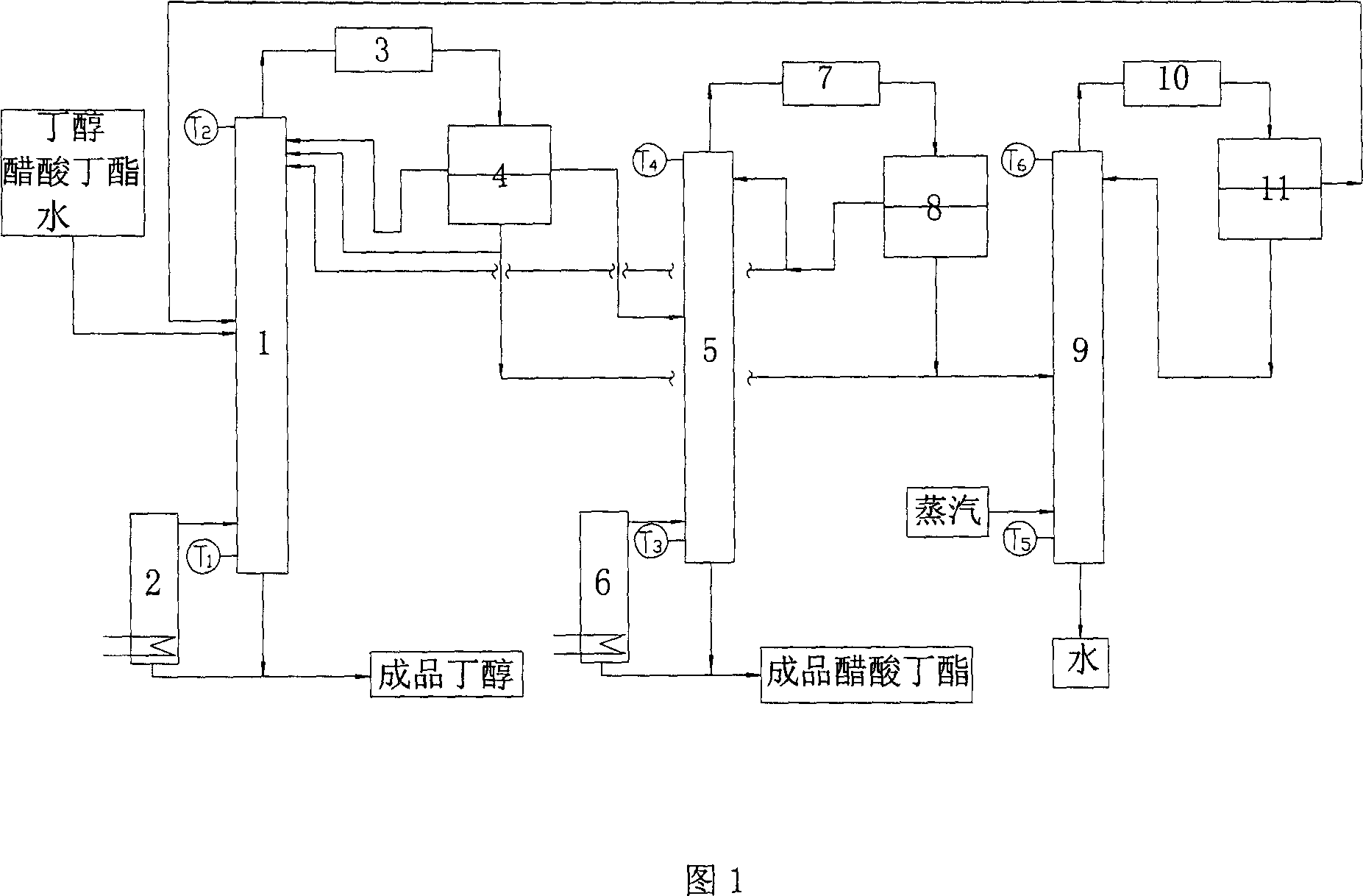
[0017] Fig. 1 is the process flow sheet of separation butanol and butyl acetate by azeotropic distillation.
[0018] Raw material solution containing butanol, butyl acetate and water 5m 3 / h, the material is fed from the middle of the rectification tower 1, the tower kettle is heated by the reboiler 2, the top product enters the phase separation tank 4 after passing through the condensation cooler 3, the heavy phase water part returns to the tower, and the light phase refluxes. Heavy phase water returns in the rectifying tower 1, the butyl acetate concentration of tower top is improved, when still temperature T 1 =123.6°C, top temperature T 2 When =90.1 DEG C, the rectification tower 1 tower tank obtains the finished product butanol. Among them, the moisture content is 0.12%, and the ester content is 3.56%. The ester content in the light phase is relatively high, and part of it is recovered and enters the rectification tower 5. The distillation column 5 is heated by a rebo...
Embodiment 2
[0020] Raw material solution 8m 3 / h, the material is fed from the middle of the rectification tower 1, the tower kettle is heated by the reboiler 2, the top product enters the phase separation tank 4 after passing through the condensation cooler 3, the heavy phase water part returns to the tower, and the light phase refluxes. Heavy phase water returns in the rectifying tower 1, the butyl acetate concentration of tower top is improved, when still temperature T 1 =123.4°C, top temperature T 2 When =90.3 DEG C, the finished product butanol was obtained in 1 tower kettle. Among them, the moisture content is 0.08%, and the ester content is 4.61%. The ester content in the light phase is relatively high, and part of it is recovered and enters the rectification tower 5. The distillation tower 5 is heated by a reboiler 6, and the top product enters the phase separation tank 8 after passing through the condensing cooler 7, and the light phase part is refluxed, and part of it is reco...
Embodiment 3
[0022] Raw material solution 10m 3 / h, the material is fed from the middle of the rectification tower 1, the tower kettle is heated by the reboiler 2, the top product enters the phase separation tank 4 after passing through the condensation cooler 3, the heavy phase water part returns to the tower, and the light phase refluxes. Heavy phase water returns in the rectifying tower 1, the butyl acetate concentration of tower top is improved, when still temperature T 1 =122.6°C, top temperature T 2 When =91.5 DEG C, 1 tower still obtains finished product butanol. Among them, the moisture content is 0.07%, and the ester content is 1.4%. The ester content in the light phase is relatively high, and part of it is recovered and enters the rectification tower 5. The distillation tower 5 is heated by a reboiler 6, and the top product enters the phase separation tank 8 after passing through the condensing cooler 7, and the light phase part is refluxed, and part of it is recovered and ret...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com