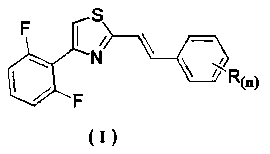
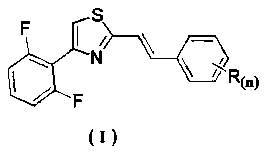
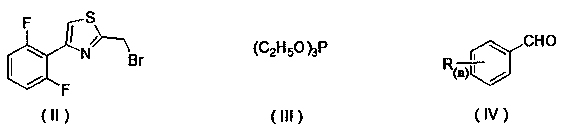Application of distyrene-like compound containing thiazole ring as sterilizing agent
A stilbene and thiazole ring-containing technology, applied in the direction of fungicides, applications, biocides, etc., can solve the problems of structure and biological activity research that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of Derivative Ia (R(n)=H):
[0030] Add 2-(bromomethyl)-4-(2,6-difluorophenyl)thiazole (2.9 g, 10 mmol) into 15 mL (86.8 mmol) of triethyl phosphite, heat to reflux, and detect the reaction by TLC , and the reaction was complete after about 1 h. Concentrate to remove excess triethyl phosphite to obtain a concentrated solution; add 20 mL of DMF, benzaldehyde (1.3 g, 12 mmol) and sodium hydroxide (0.9 g, 22 mmol) to the obtained concentrated solution to react. The reaction process was detected by TLC, and the reaction was completed in about 3 hours. The reaction solution was poured into 150 mL of ice water, stirred, and a solid precipitated, filtered, and then recrystallized with ethyl acetate to obtain 1.6 g of a yellow solid, which was (E)-4 -(2,6-difluorophenyl)-2-styrylthiazole, the calculated yield is 54.7%. m.p.: 79~80℃;
[0031] 1 H NMR (500 MHz, Chloroform- d ) δ 7.56 (d, J = 7.5 Hz, 2H), 7.50 – 7.37(m, 5H), 7.37 – 7.26 (m, 2H), 7.02 (t,...
Embodiment 2
[0033] Example 2 Synthesis of derivative Ib (R(n)=o-chloro):
[0034] Add 2-(bromomethyl)-4-(2,6-difluorophenyl)thiazole (2.9 g, 10 mmol) into 9.0 mL (50.0 mmol) triethyl phosphite, heat to reflux, and detect the reaction by TLC , and the reaction was completed after about 1.5 h. Concentrate to remove excess triethyl phosphite to obtain a concentrated solution; add 25 mL of DMF, o-chlorobenzaldehyde (1.4 g, 10 mmol) and sodium hydroxide (1.3 g, 32 mmol) to the obtained concentrated solution for reaction. The reaction process was detected by TLC, and the reaction was completed in about 3.5 hours. The reaction solution was poured into 150 mL of ice water, stirred, and a solid was precipitated, filtered, and then recrystallized with ethanol to obtain 1.4 g of a yellow solid, which was (E)-4-( 2,6-difluorophenyl)-2-o-chlorostyrylthiazole, the calculated yield is 40.6%. m.p.: 87~89℃;
[0035] 1 H NMR (500 MHz, Chloroform- d ) δ 7.83 (d, J = 16.0 Hz, 1H), 7.74 – 7.67(dd, J ...
Embodiment 3
[0037] Example 3 Synthesis of derivative Ic (R(n)=p-chloro):
[0038] Add 2-(bromomethyl)-4-(2,6-difluorophenyl)thiazole (2.9 g, 10 mmol) into 12 mL (69.5 mmol) of triethyl phosphite, heat to reflux, and detect the reaction by TLC , and the reaction was completed after about 3 h. Concentrate to remove excess triethyl phosphite to obtain a concentrated solution; add 22 mL of DMF, p-chlorobenzaldehyde (2.8 g, 20 mmol) and sodium hydroxide (2.0 g, 50 mmol) to the obtained concentrated solution to react. The reaction process was detected by TLC, and the reaction was completed in about 2.5 hours. The reaction solution was poured into 150 mL of ice water, stirred, and a solid was precipitated, filtered, and then recrystallized with n-hexane to obtain 1.9 g of a yellow solid, namely (E)-4- (2,6-difluorophenyl)-2-p-chlorostyrylthiazole, the calculated yield is 56.1%. m.p.: 91~93℃;
[0039] 1 H NMR (500 MHz, Chloroform- d ) δ 7.50 – 7.45 (m, 3H), 7.40 (d, J = 16.0Hz, 1H), 7.38 –...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com