Lentivirus freeze-drying protective agent and lentivirus freeze-dried powder
A technology of drying protective agent and lentivirus, which is applied in the preservation of microorganisms, microorganisms, biochemical equipment and methods, etc., can solve the problem of no lentivirus freeze-dried powder, etc., and achieve the effect of good application value, reduced activity loss, and easy transportation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of lentiviral freeze-drying protective agent
[0055] (1) Weigh an appropriate amount of disodium hydrogen phosphate and sodium dihydrogen phosphate powder, fully mix and dissolve with deionized water, adjust the pH value to 7.0-7.6 with 2M sodium hydroxide solution, and filter with a 0.45µm filter membrane to obtain phosphate Buffer solution (50mmol / L, pH7.0~7.6) at room temperature for later use.
[0056] (2) Weigh glycerin, sucrose, mannitol, L-arginine, glycine, PEG6000, and gelatin according to the following ingredients and final concentration requirements, add them to phosphate buffer, mix and dissolve thoroughly, and measure the pH value of the solution again and adjust Between 7.0 and 7.6, filter with a 0.22 μm filter membrane to obtain a lentiviral freeze-dried protective agent.
[0057] Protective agent components and content:
[0058] Glycerin 1.0% (V / V)
[0059] Sucrose 20mg / mL
[0060] Mannitol 25mg / mL
[0061] L-Arginine 1mg / mL ...
Embodiment 2
[0066] Example 2 Preparation of lentivirus freeze-dried powder
[0067] (1) The present invention prepares RCR-deficient lentivirus stock solution according to conventional methods: prepare auxiliary packaging plasmid (pspax2), envelope plasmid (pMD), expression plasmid containing GFP protein (pCDHGFP) and 293T cells; prepare the three plasmids Mix according to a certain ratio (pspax2:pMD:pCDHGFP=2:1:3) and transfect into 293T cells with PEI reagent; after 12h, replace the fresh medium to continue culturing, collect the supernatant at 48h and 72h respectively to obtain the stock solution containing lentivirus and Filtered through a 0.45 µm membrane filter and stored at 4°C for later use.
[0068](2) The present invention uses anion exchange chromatography to purify the lentivirus stock solution: weigh an appropriate amount of tris base, and prepare equilibration buffer A (25mM Tris, pH8.0) and elution buffer B (1M Tris, pH 8.0) with deionized water, respectively. pH8.0), take...
Embodiment 3
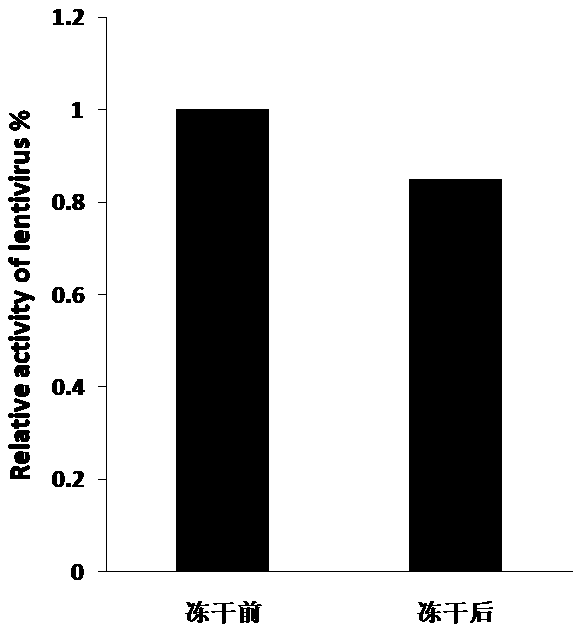
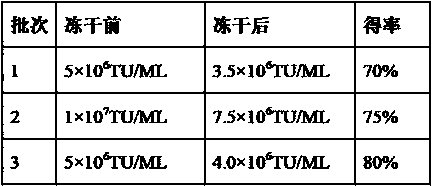
[0071] Example 3 Comparative analysis of activity of lentiviral freeze-dried powder
[0072] According to the formula described in Example 1 and the production process described in Example 2, three batches of lentivirus lyophilized powder were produced, and the transduction activity (TU / ML) was measured before and after lyophilization. The test results are as follows:
[0073] Table 1 The results of transduction activity determination of lentiviral lyophilized powder before and after lyophilization
[0074]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap