Method for synthesizing alpha-amino acid
A synthesis method and amino acid technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as harsh conditions, achieve cheap and easy-to-obtain raw materials, and avoid the effect of subsequent purification operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
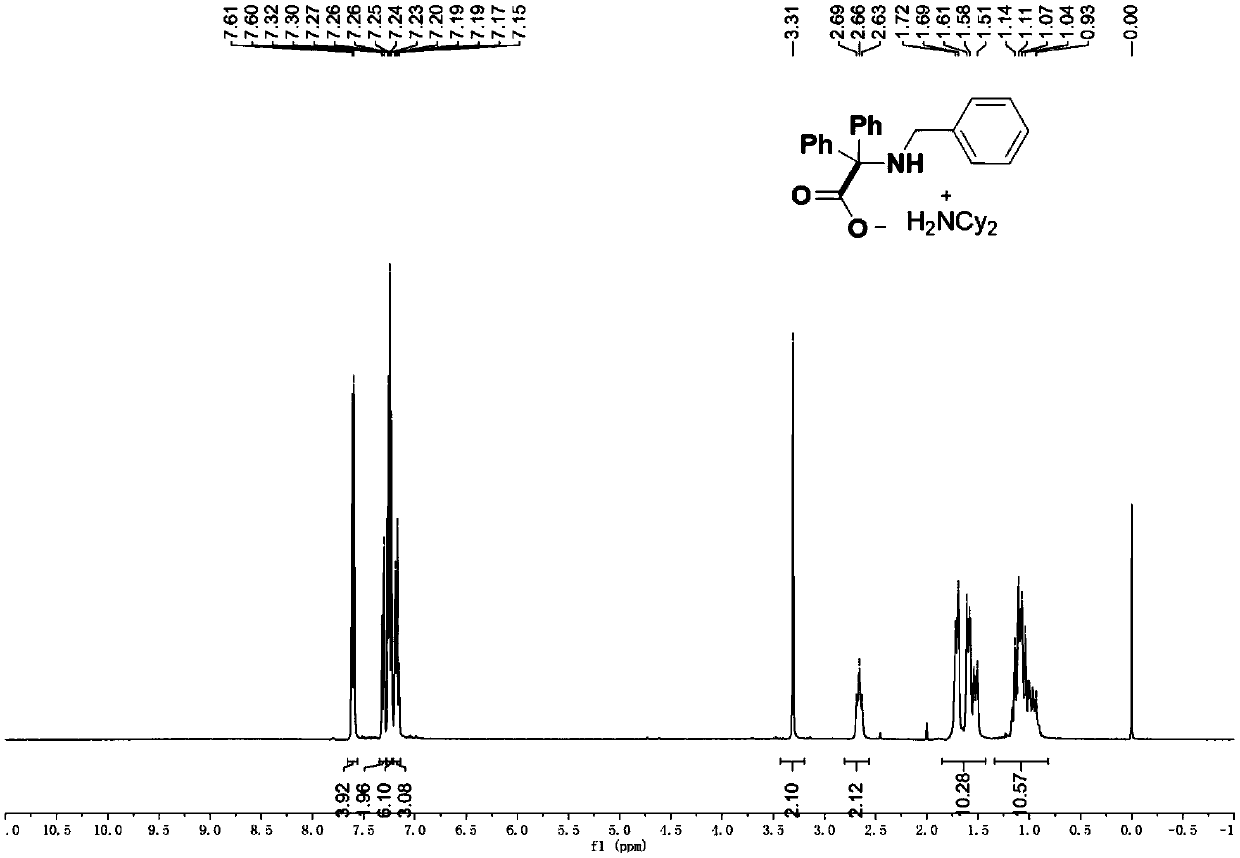
[0063] 2-(Benzylamino)-2,2-diphenylacetic acid dicyclohexylamine salt (4a).
[0064]
[0065] According to the typical experimental procedure, with ketimine N-benzyl-1,1-diphenylmethanimine (54.2 mg, 0.2 mmol), catalyst iridium-7 (0.9 mg, 0.5mol%), N, N- Dicyclohexylmethylamine (85.6 μL, 0.4 mmol), carbon dioxide (in balloon), 2 mL of acetonitrile, and the reaction was performed. After the reaction, the target product was obtained by filtration, which was a white solid with a melting point of 154.9-155.5°C (90.8 mg, yield 91%).
[0066] 1H NMR (400MHz, CDCl3): δ7.60(d, J=7.2Hz, 4H), 7.31(d, J=7.0Hz, 2H), 7.28-7.22(m, 6H), 7.21-7.14(m, 3H ), 3.31 (s, 2H), 2.66 (t, J=10.8Hz, 2H, Cy), 1.85-1.43 (m, 10H, Cy), 1.34-0.81 (m, 10H, Cy) ppm;
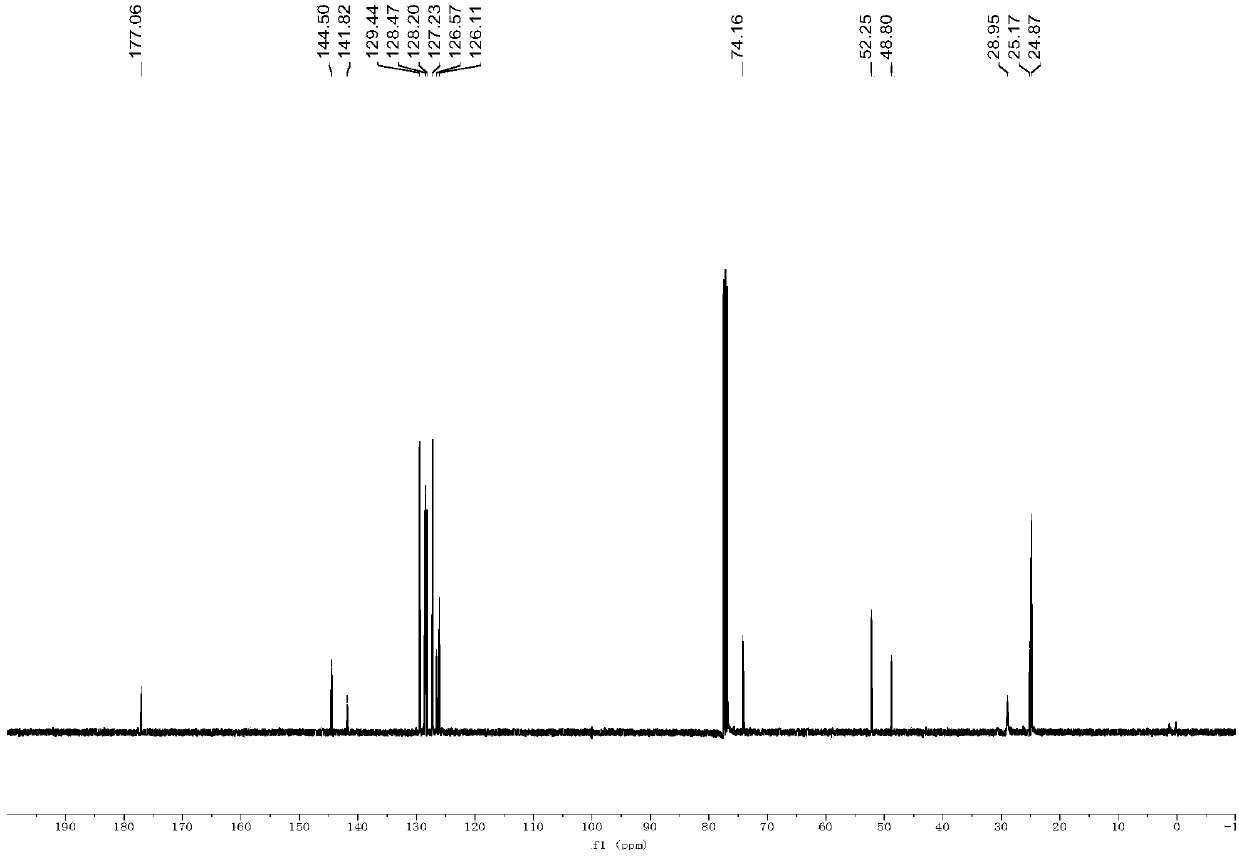
[0067] 13C{1H}NMR (100MHz, CDCl3): δ177.06, 144.50, 141.82, 129.44, 128.47, 128.20, 127.23, 126.57, 126.11, 74.16, 52.25(Cy), 48.80, 28.95(Cy), 25.17(Cy), 24.87 (Cy) ppm;
[0068] IR (thin film): 2928, 2853, 1632, 1360, 1347, 1117, 1098, 7...
Embodiment 2
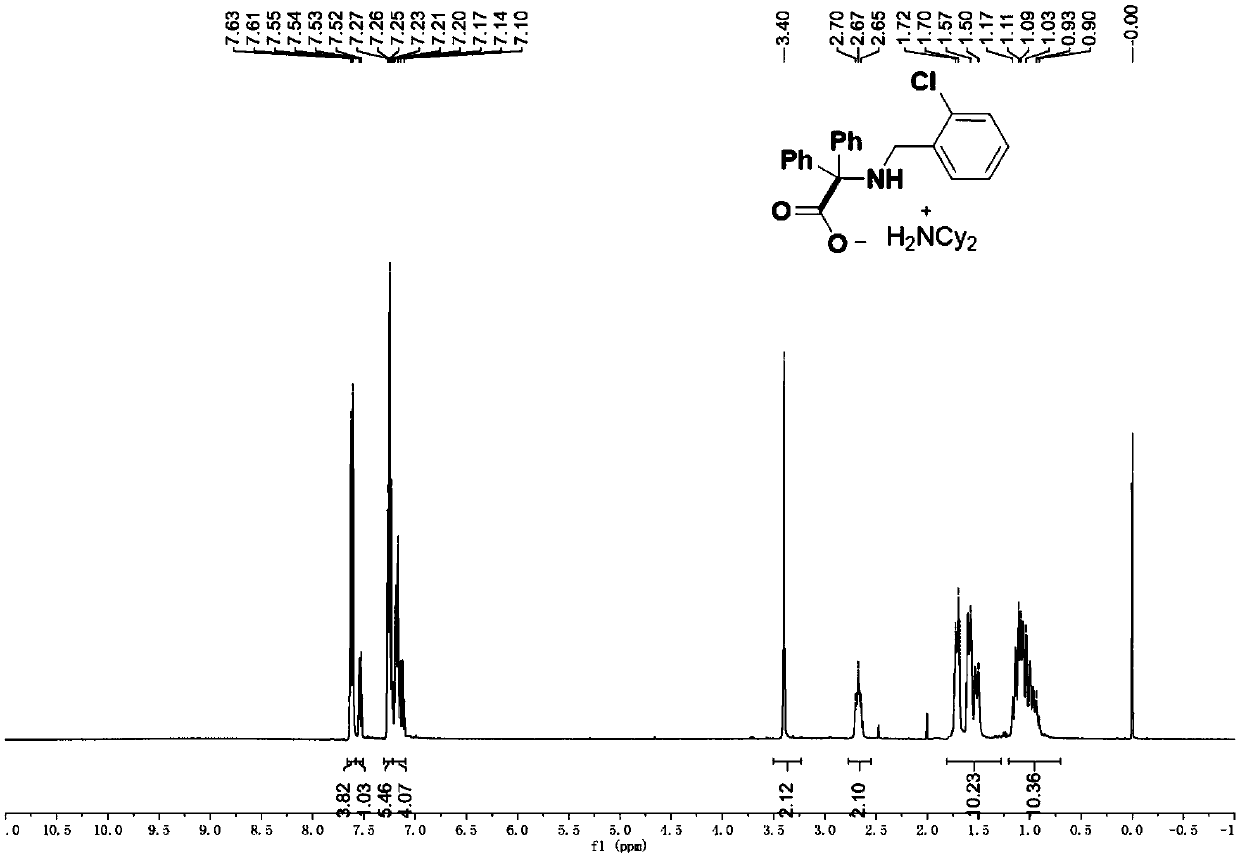
[0071] 2-((2-chlorobenzyl)amino)-2,2-diphenylacetic acid dicyclohexylamine salt (4b)
[0072]
[0073] According to the typical experimental procedure, with ketimine N-(2-chlorobenzyl)-1,1-diphenylmethanimine (61.2 mg, 0.2 mmol), catalyst iridium-7 (0.9 mg, 0.5mol%) , N,N-Dicyclohexylmethylamine (85.6 μL, 0.4 mmol), carbon dioxide (in balloon), 2 mL of acetonitrile, and the reaction was carried out. After the reaction, the target product was obtained by filtration, which was a white solid with a melting point of 138.5-139.6°C (94.9 mg, yield 89%).
[0074] 1H NMR (400MHz, CDCl3): δ7.62(d, J=7.2Hz, 4H), 7.53(dd, J=7.6, 1.7Hz, 1H), 7.30-7.22(m, 5H), 7.22-7.09(m , 4H), 3.40 (s, 2H), 2.67 (t, J=10.9Hz, 2H, Cy), 1.81-1.28 (m, 10H, Cy), 1.20-0.70 (m, 10H, Cy) ppm;
[0075] 13C{1H}NMR (100MHz, CDCl3): δ176.85, 144.24, 139.34, 133.57, 129.96, 129.23, 128.97, 127.54, 127.08, 126.58, 125.96, 73.90, 52.09(cy), 45.89, 28.61( (cy), 24.71 (cy) ppm.ppm;
[0076] IR (thin film): 2940, ...
Embodiment 3
[0079] 2-((3-chlorobenzyl)amino)-2,2-diphenylacetic acid dicyclohexylamine salt (4c)
[0080]
[0081] According to the typical experimental procedure, with ketimine N-(3-chlorobenzyl)-1,1-diphenylmethanimine (61.2 mg, 0.2 mmol), catalyst iridium-7 (0.9 mg, 0.5mol%) , N,N-Dicyclohexylmethylamine (85.6 μL, 0.4 mmol), carbon dioxide (in balloon), 2 mL of acetonitrile, and the reaction was carried out. After the reaction, the target product was obtained by filtration, which was a white solid with a melting point of 148.0-149.6°C (96 mg, yield 90%).
[0082] 1H NMR (400MHz, CDCl3): δ7.58(d, J=7.4Hz, 4H), 7.36-7.11(m, 10H), 3.27(s, 2H), 2.66(brs, 2H, Cy), 1.84-1.45 (m, 10H, Cy), 1.16-0.93 (m, 10H, Cy) ppm;
[0083] 13C{1H}NMR (100MHz, CDCl3): δ176.95, 144.33, 144.05, 133.98, 129.41(x2), 128.42, 127.28, 126.67, 126.59, 126.21, 74.08, 52.28(Cy), 48.39, 28.90(Cy), 25.17(Cy), 24.85(Cy)ppm;
[0084] IR (thin film): 2935, 1627, 1551, 1447, 1358, 1349, 810, 705cm-1;
[0085] HRMS ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com