Tretinoin crystal form A and preparation method thereof
A technology of retinoic acid and crystal form, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Preparation of Retinoic Acid Crystal Form A
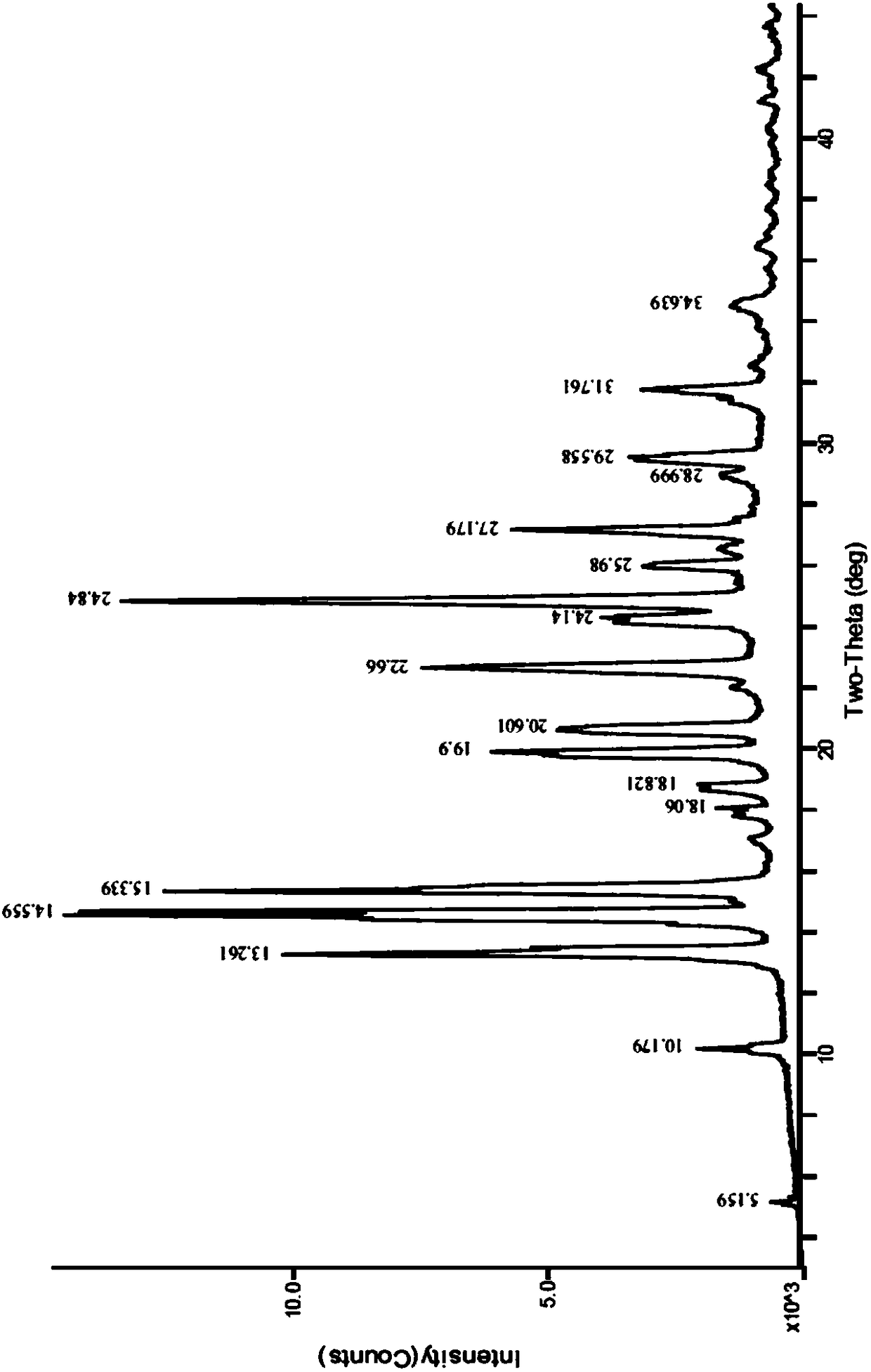
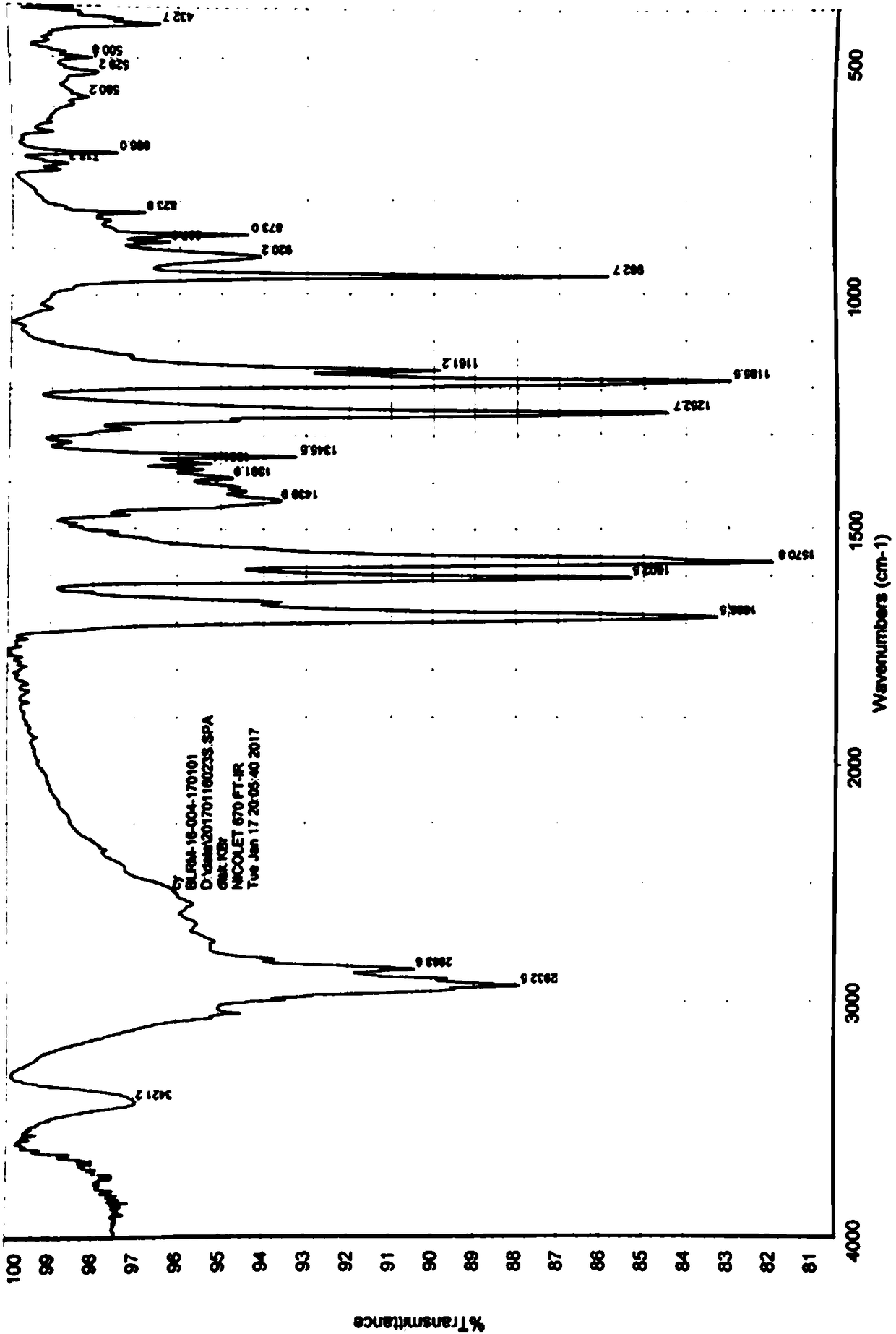
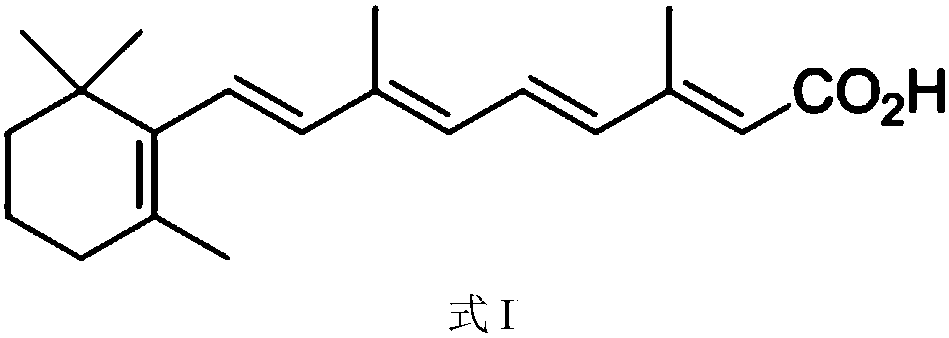
[0032] Weigh 5.0 g of tretinoin (prepared with reference to the method disclosed in CN102558007A, the same below), add 50 ml of ethyl acetate, heat to reflux to dissolve, cool naturally to room temperature while stirring, precipitate solids, and continue cooling to 0-5°C , and then keep warm for 1 hour. Filter and dry to obtain light yellow crystalline powder with a yield of 85%. The crystalline powder is confirmed as retinoic acid crystal form A by XRPD, and the X-ray powder diffraction pattern is as attached figure 1 As shown, the infrared spectrum is attached as figure 2 shown.
Embodiment 2
[0033] Preparation of Example 2 Retinoic Acid Crystal Form A
[0034] Weigh 5.0g tretinoin, add 100ml of methanol, heat to reflux to dissolve, then cool naturally to room temperature with stirring, precipitate solid, continue to cool to 0-5°C, and keep warm for 1 hour. Filter and dry to obtain a yellow crystalline powder, which is confirmed as retinoic acid crystal form A by XRPD, with a yield of 78%.
Embodiment 3
[0035] Preparation of embodiment 3 dimension A acid crystal form A
[0036] Weigh 5.0g tretinoin, add 100ml of ethanol, heat to reflux to dissolve, then cool naturally to room temperature while stirring, and solid precipitates, continue cooling to 0-5°C, and keep warm for 1 hour. Filter and dry to obtain a yellow crystalline powder, which is confirmed as retinoic acid crystal form A by XRPD, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com