Dihydrogen phosphate ionic liquid promotes the method for synthesizing dihydropyrimidinone compounds
A technology of dihydropyrimidinones and dihydrogen phosphate, applied in the field of synthesis of dihydropyrimidinones, to achieve the effects of simple preparation process, high catalytic activity, simple operation and post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
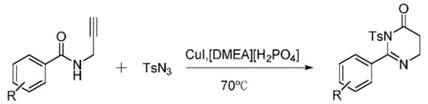
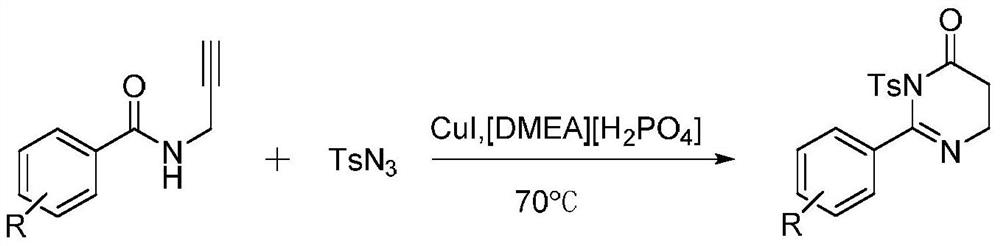
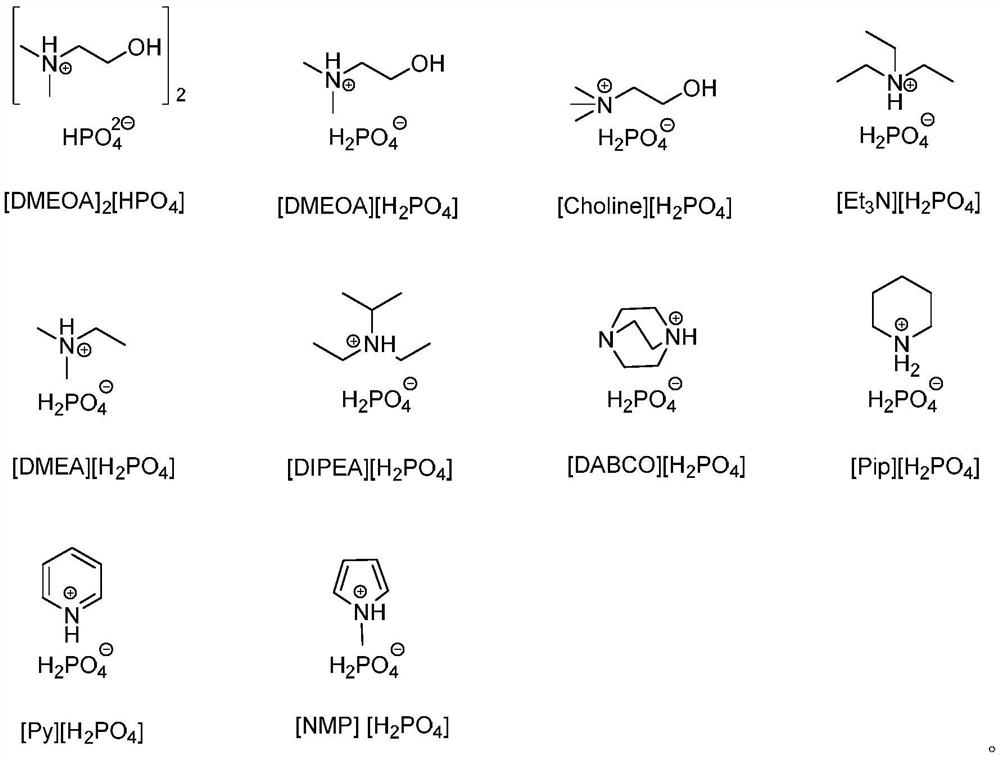
[0018] Add 0.05mmol [DMEA][H 2 PO 4 ] and 0.01mol CuI, 0.1mmol N-propargyl benzamide and 0.12mmol p-toluenesulfonyl azide, stirred and mixed on a magnetic stirrer, and then placed the reaction flask in an oil bath at 70 ° C to heat and stir the reaction 30min, the whole reaction process is tracked and monitored with TLC, after the reaction finishes, extract the reaction mixture with secondary water and dichloromethane, combine the dichloromethane phases, distill under reduced pressure and spin-dry the solvent, and the crude product is recrystallized (normal hexane: ethyl acetate Ester=7:1, v / v) to obtain the target product with a yield of 78%.
Embodiment 2
[0020] Add 0.05mmol [DMEA][H 2 PO 4 ] and 0.01mol CuI, 0.1mmol N-propargyl-4-methylbenzamide and 0.12mmol p-toluenesulfonyl azide, stirred and mixed on a magnetic stirrer, and then placed the reaction flask in an oil bath at Heated and stirred at 70°C for 30 min. The entire reaction process was tracked and monitored by TLC. After the reaction, the reaction mixture was extracted with secondary water and dichloromethane, the dichloromethane phase was combined, the solvent was distilled and spin-dried under reduced pressure, and the crude product was recrystallized ( n-hexane:ethyl acetate=7:1, v / v) to obtain the target product with a yield of 82%.
Embodiment 3
[0022] Add 0.05mmol [DMEA][H 2 PO 4 ] and 0.01mol CuI, 0.1mmol N-propargyl-3-methylbenzamide and 0.12mmol p-toluenesulfonyl azide, stirred and mixed on a magnetic stirrer, and then placed the reaction flask in an oil bath at Heated and stirred at 70°C for 30 min. The entire reaction process was tracked and monitored by TLC. After the reaction, the reaction mixture was extracted with secondary water and dichloromethane, the dichloromethane phase was combined, the solvent was distilled and spin-dried under reduced pressure, and the crude product was recrystallized ( n-Hexane:ethyl acetate=7:1, v / v) to obtain the target product with a yield of 80%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap