Combination Therapies for the Treatment of Malignancies
A utility, acute technique in the field of combination therapy for the treatment of malignant tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
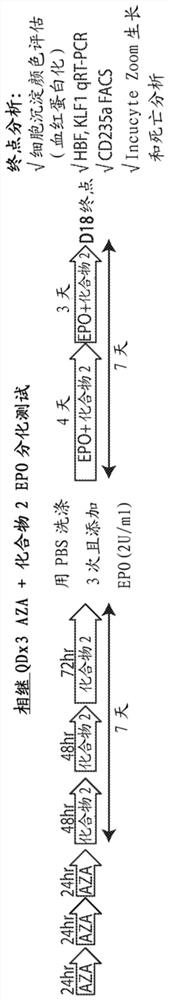
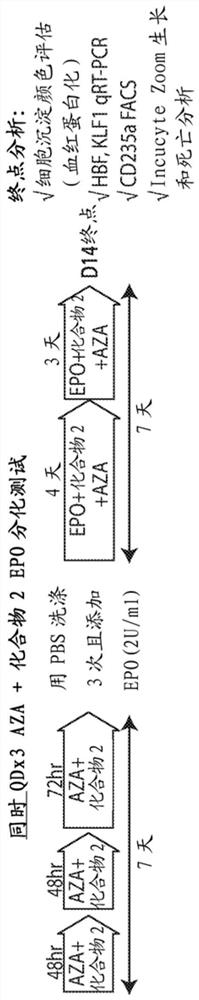
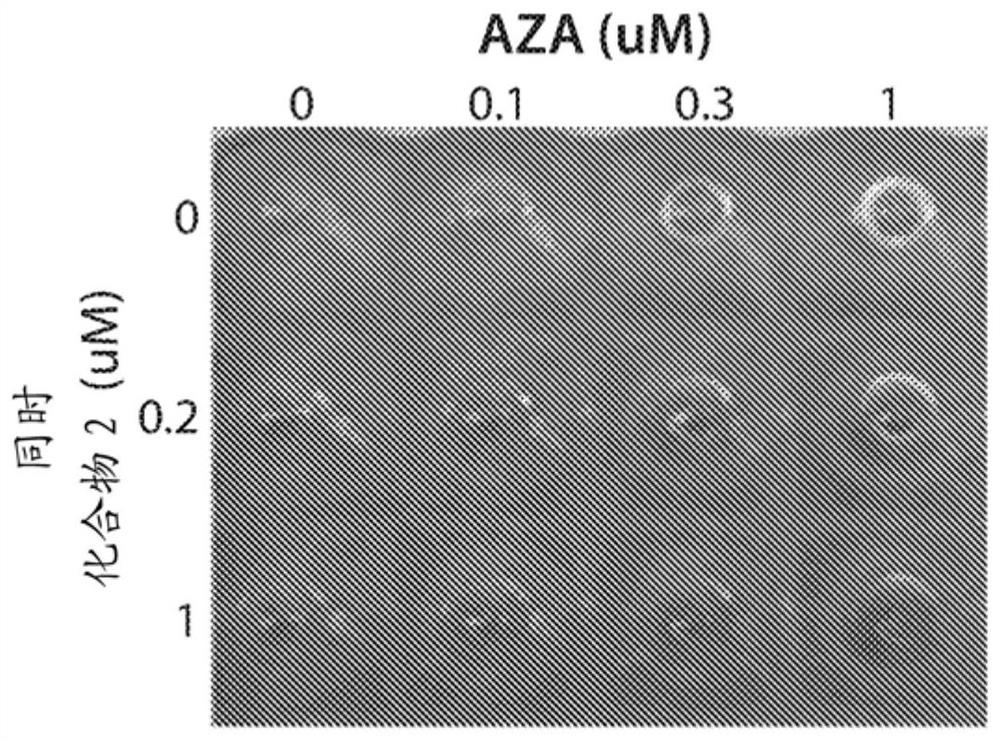
[0303] Example 1. Effect of the combination of compound 2 and azacitidine on EPO-differentiation in AML cells
[0304] TF1-IDH1 R132H EPO differentiation assay in cells. in TF1-IDH1 R132H Measures of cell differentiation, growth and death were assessed in cells using the in vitro EPO differentiation assay and the dose-schedule paradigm shown in Figure 1 . Cells were treated with vehicle, AZA alone, Compound 2 alone or the combination of AZA+Compound 2. In the sequential protocol, cells were pretreated with AZA for three days, followed by the addition of Compound 2. In the simultaneous protocol, cells were co-treated with AZA and Compound 2 throughout the test. Endpoint assessment assays were: assessment of cell pellet color (hemoglobinization test); HBG and KLF1 RNA by RT-qPCR; CD235a-positive cell population by flow cytometry (marker of differentiation); and growth and apoptosis by IncuCyteZoom real-time imaging.
[0305] Compounds: Compound 2 was used as a 10 mM stock ...
Embodiment 2
[0323] Example 2. Phase 1b / 2 open-label randomized study of 2 combinations of isocitrate dehydrogenase (IDH) mutant-targeted therapy and azacitidine: oral compound 2 plus subcutaneous azacitidine and oral Compound 1 plus subcutaneous azacitidine in newly diagnosed acute myeloid leukemia subjects carrying IDH1 or IDH2 mutations, respectively, who are not candidates for intensive induction chemotherapy
[0324] Indications: For the treatment of subjects 18 years of age or older with newly diagnosed acute myeloid leukemia (AML) carrying IDH1 or IDH2 mutations, respectively, who are not candidates for intensive induction chemotherapy
[0325] Treatment of newly diagnosed acute myeloid leukemia (AML) patients over 18 years of age with IDH1 or IDH2 mutations who are not candidates for intensive induction chemotherapy (IC).
[0326] Key Target - Phase 1b (Dose Escalation Phase)
[0327] main target
[0328] To evaluate oral compound 2 plus subcutaneous (SC) azacitidine and oral 2-m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com