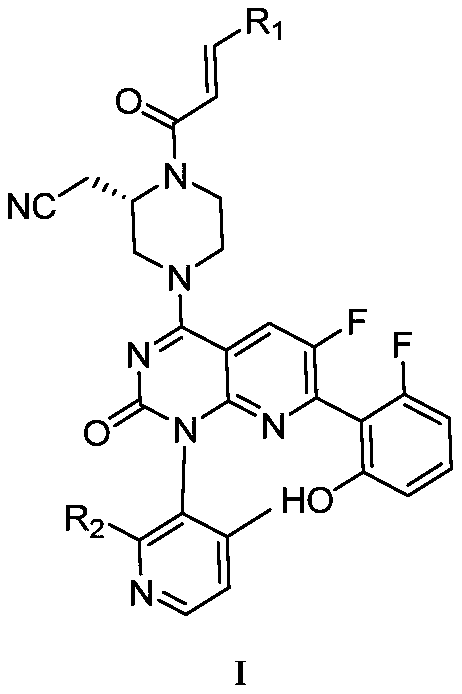
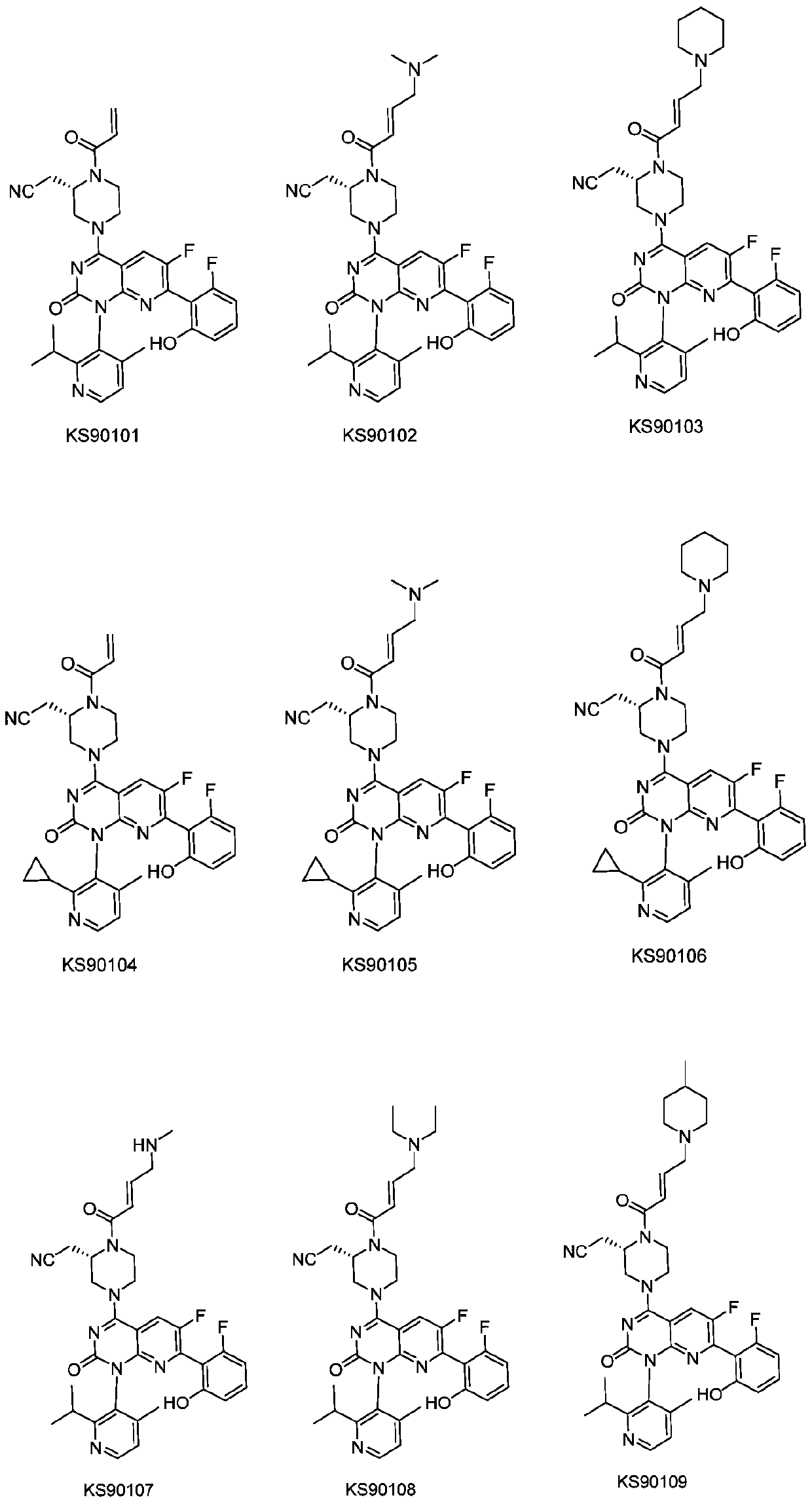
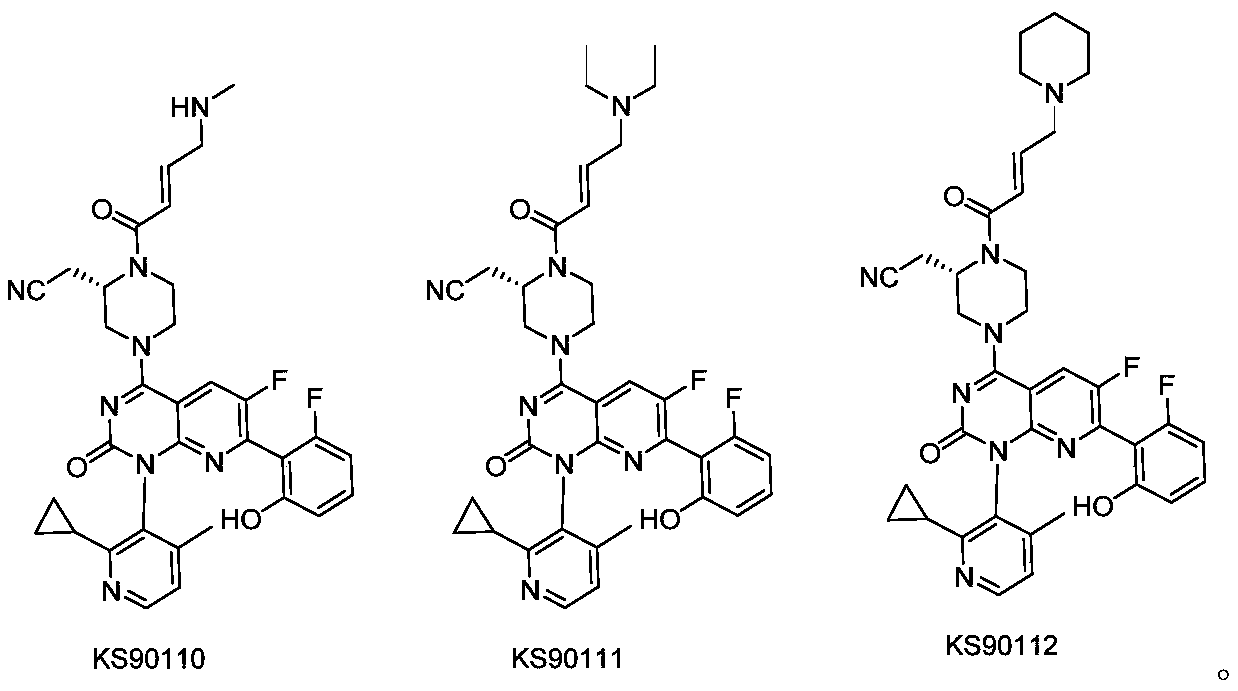
Nitrile methyl piperazine derivative serving as KRAS G12C mutant protein inhibitor and application of nitrile methyl piperazine derivative
A solvate and alkyl technology, which is applied in the field of preparation of cancer drugs, can solve the problems of difficulty in further improving activity, no performance activity, poor metabolic stability, etc., and achieve significant anti-tumor activity, high selectivity, Effects of High Exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1KS90100-K1-A
[0049]
[0050] step 1:
[0051] 2,6-Dichloro-5-fluoronicotinic acid (20.0g, 95.2mmol) was dissolved in dichloromethane (200mL), cooled to 0°C, oxalyl chloride (24.20g, 19.6mmol) was added dropwise, and raised to React overnight at room temperature, and take a sample to analyze that the reaction is complete. The reaction was concentrated, dissolved by adding 1,4-dioxane (50mL), cooled to 0°C, added dropwise to ammonia water (50mL), added dropwise, raised to room temperature and stirred for 3 hours, then sampled and analyzed for complete reaction. The reaction liquid was quenched with water, extracted with ethyl acetate, combined organic phases, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated, and the crude product was purified by silica gel column chromatography to obtain KS90100-K1-A1 (19.8 g, yield 99% %).
[0052] Step 2:
[0053] 2-Bromo-3-amino-4-methylpyridine ...
Embodiment 2
[0062] The synthesis of embodiment 2KS90100-K1-B
[0063]
[0064] Referring to the preparation method of Example 1, KS90100-K1-B (crude product) was obtained, which was directly used in the next step. The synthesis of embodiment 3KS90100-K2
Embodiment 3
[0064] Referring to the preparation method of Example 1, KS90100-K1-B (crude product) was obtained, which was directly used in the next step. The synthesis of embodiment 3KS90100-K2
[0065]
[0066] step 1:
[0067] (R)-Piperazine-2-carboxylate hydrochloride (5.0g, 24.6mmol) was dissolved in water (10mL) and 1,4-dioxane (40mL), cooled to 0°C, and NaOH (2.96 g, 74.0mmol) in water (6mL), stir well; add (Boc) 2 O (11.3g, 51.8mmol), stirred at room temperature for 20 hours, the reaction was complete. The pH of the reaction solution was adjusted to 5 with 1M dilute hydrochloric acid, extracted with ethyl acetate, the organic phases were combined, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated, and the crude product was beaten with PE to obtain a white solid KS90100-K2-1 (7.5g , yield 92%). LC-MS: m / z=329.2 [M-H] -
[0068] Step 2:
[0069] At 0°C, add thionyl chloride (13.2g, 111mmol) dropwise to DMF (8.47g, 116mmol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com