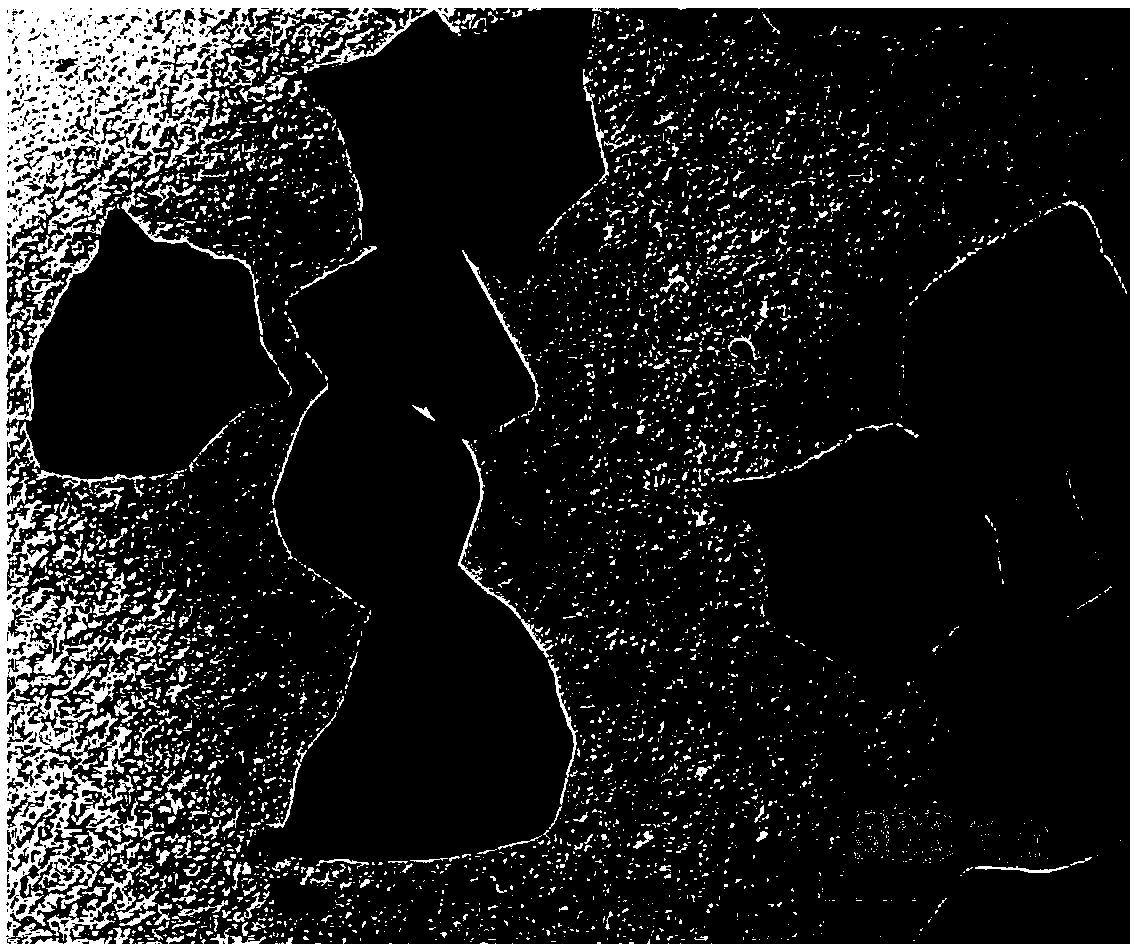
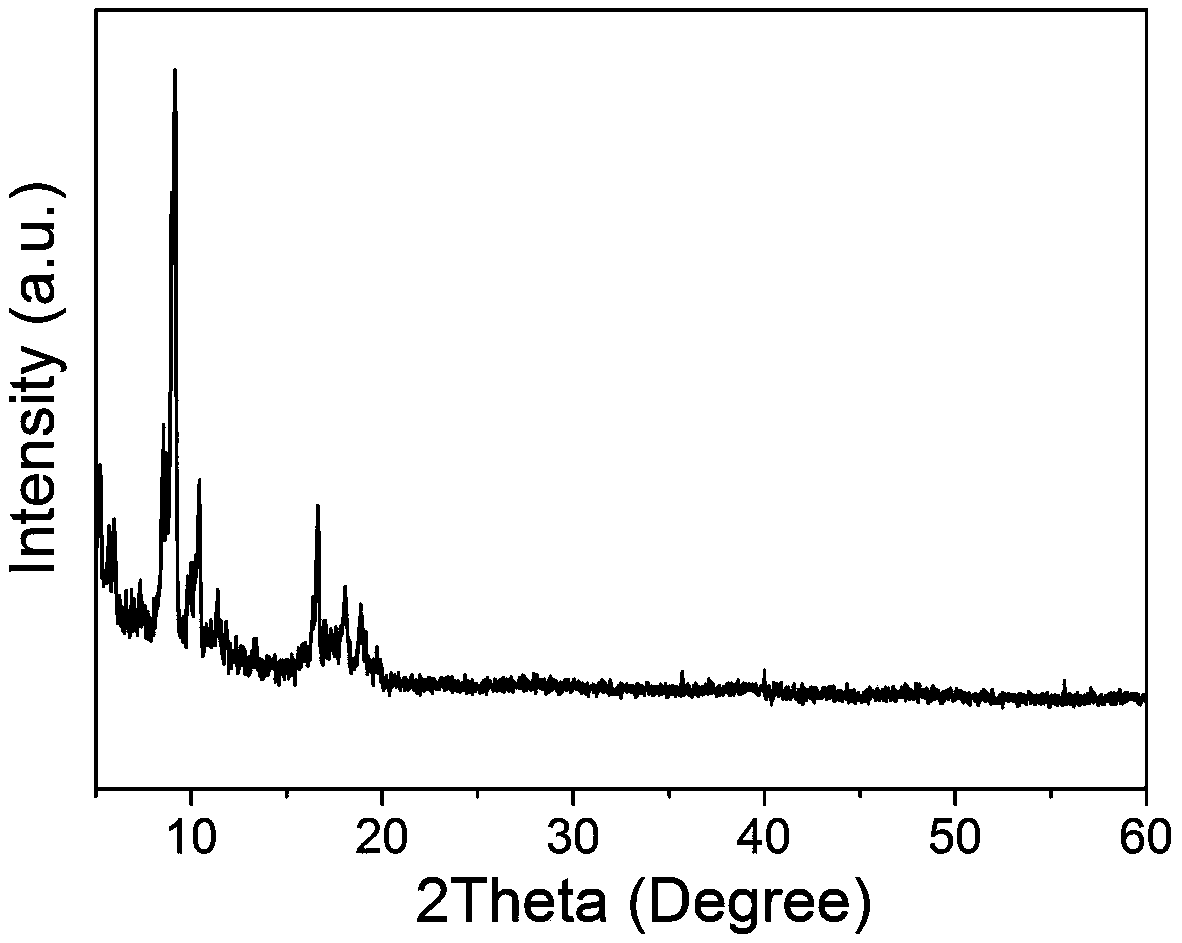
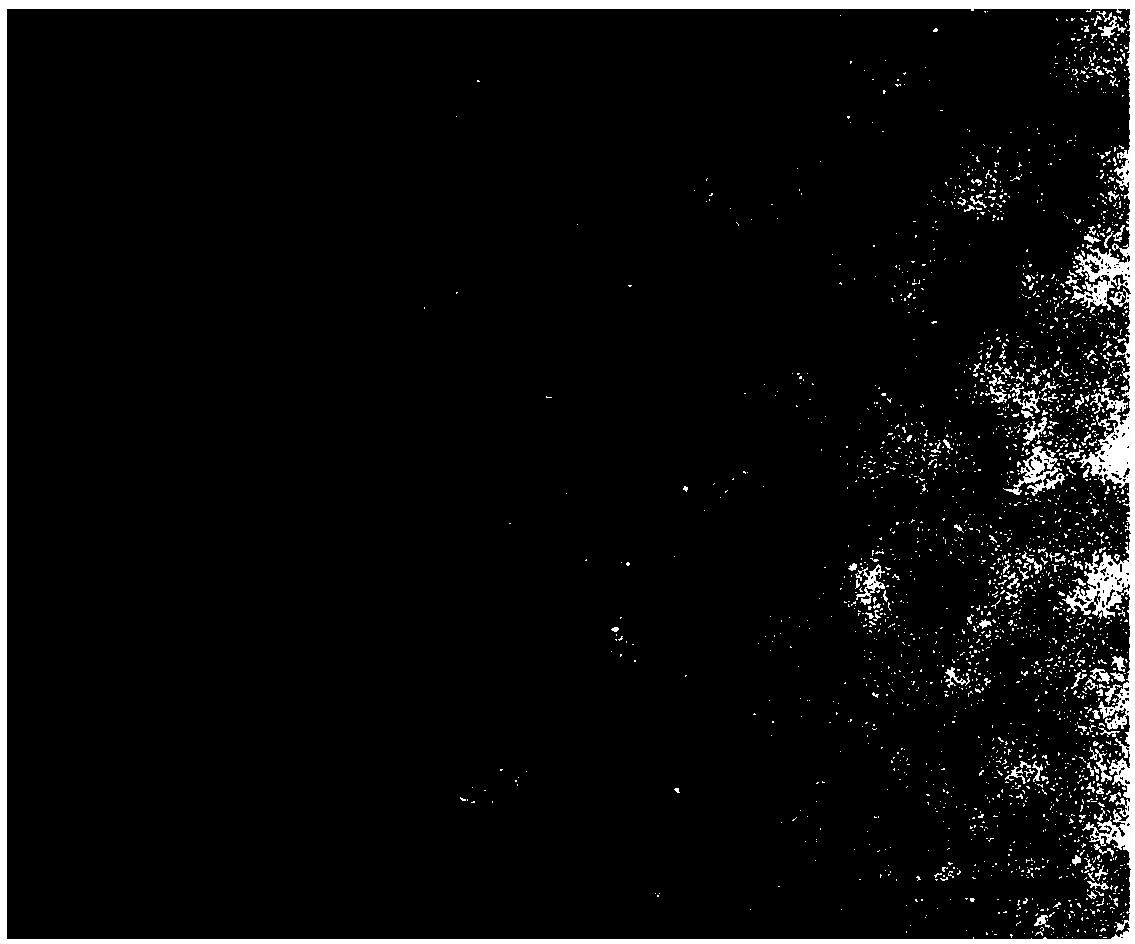
Pt1@MIL nano-catalyst, and preparation method and application thereof
A nano-catalyst and polar solvent technology, applied in the field of Pt1@MIL nano-catalyst and its preparation, can solve the problems of low energy density of gaseous products, high cost of separation, storage and transportation, and achieve low cost, mild conditions and simple procedures Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0058] The present invention Pt 1 The preparation method of @MIL nano-catalyst comprises the following steps:
[0059] (1) Uniformly disperse the metal-organic framework MIL-101 nanoparticles obtained above in deionized water to obtain a mixed solution A; wherein, the mass-volume ratio (mg / mL) of the metal-organic framework MIL-101 to deionized water is 95:65 ;
[0060] (2) Slowly add potassium tetrachloroplatinate solution and sodium borohydride solution to mixed solution A and mix uniformly to obtain mixed solution B; wherein, the concentration of potassium tetrachloroplatinate solution is 0.03mg / mL, sodium borohydride solution The concentration is 0.003mg / mL, the volume ratio of potassium tetrachloroplatinate solution and deionized water is 10:60, and the volume ratio of sodium borohydride solution and deionized water is 10:60;
[0061] (3) Stir the mixed solution B at room temperature for 4 hours, and wash the stirred product. The specific operation is as follows: centri...
specific Embodiment approach 2
[0064] The present invention Pt 1 The preparation method of @MIL nano-catalyst comprises the following steps:
[0065] (1) The metal-organic framework MIL-101 nanoparticles obtained above were uniformly dispersed in deionized water to obtain a mixed solution A; wherein, the mass-volume ratio (mg / mL) of the metal-organic framework MIL-101 to deionized water was 105:70 ;
[0066] (2) Slowly add potassium tetrachloroplatinate solution and sodium borohydride solution to mixed solution A and mix uniformly to obtain mixed solution B; wherein, the concentration of potassium tetrachloroplatinate solution is 0.05mg / mL, sodium borohydride solution The concentration is 0.005mg / mL, the volume ratio of potassium tetrachloroplatinate solution and deionized water is 10:60, and the volume ratio of sodium borohydride solution and deionized water is 10:60;
[0067] (3) Stir the mixed solution B at room temperature for 7 hours, and wash the stirred product. The specific operation is as follows...
specific Embodiment approach 3
[0070] The present invention Pt 1 The preparation method of @MIL nano-catalyst comprises the following steps:
[0071] (1) Uniformly disperse the metal-organic framework MIL-101 nanoparticles obtained above in deionized water to obtain a mixed solution A; wherein, the mass-volume ratio (mg / mL) of the metal-organic framework MIL-101 to deionized water is 100:60 ;
[0072] (2) Slowly add potassium tetrachloroplatinate solution and sodium borohydride solution to mixed solution A and mix uniformly to obtain mixed solution B; wherein, the concentration of potassium tetrachloroplatinate solution is 0.04mg / mL, and sodium borohydride solution The concentration is 0.004mg / mL, the volume ratio of potassium tetrachloroplatinate solution and deionized water is 10:60, and the volume ratio of sodium borohydride solution and deionized water is 10:60;
[0073] (3) Stir the mixed solution B at room temperature for 5 hours, and wash the stirred product. The specific operation is as follows: c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com