Hydroxy substituted tetrahydro-beta-carboline small-molecular organic compounds, as well as derivatives and medical application thereof
A technology of organic compounds and small molecules, applied in the field of medicine, can solve the problems of poor physical and chemical properties, not being widely used, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
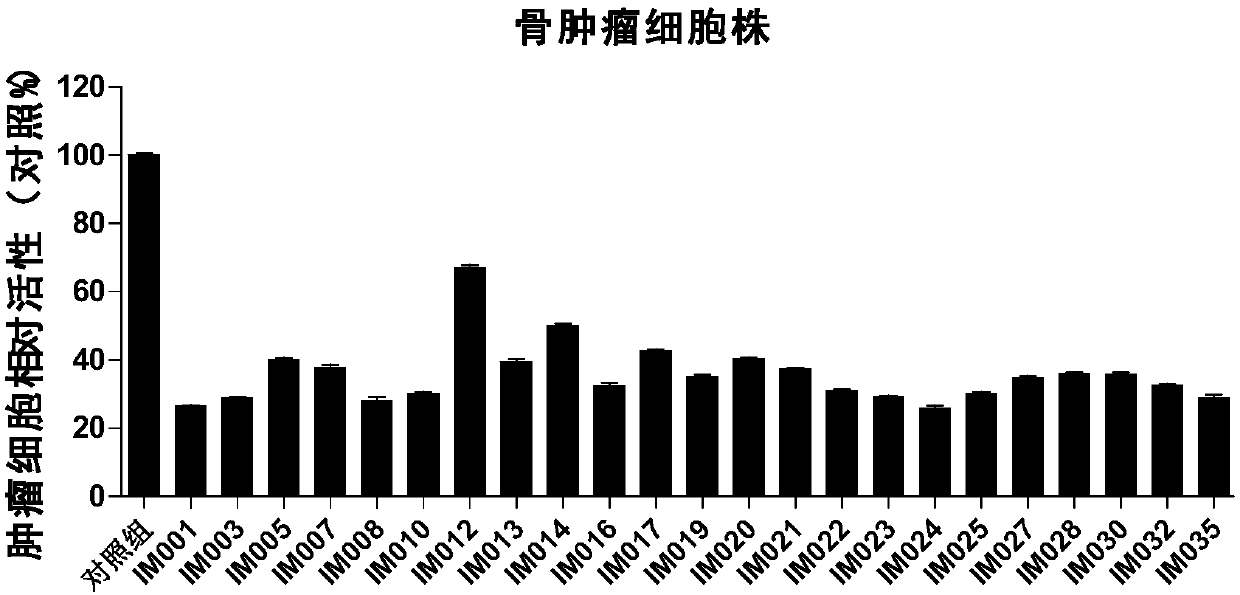
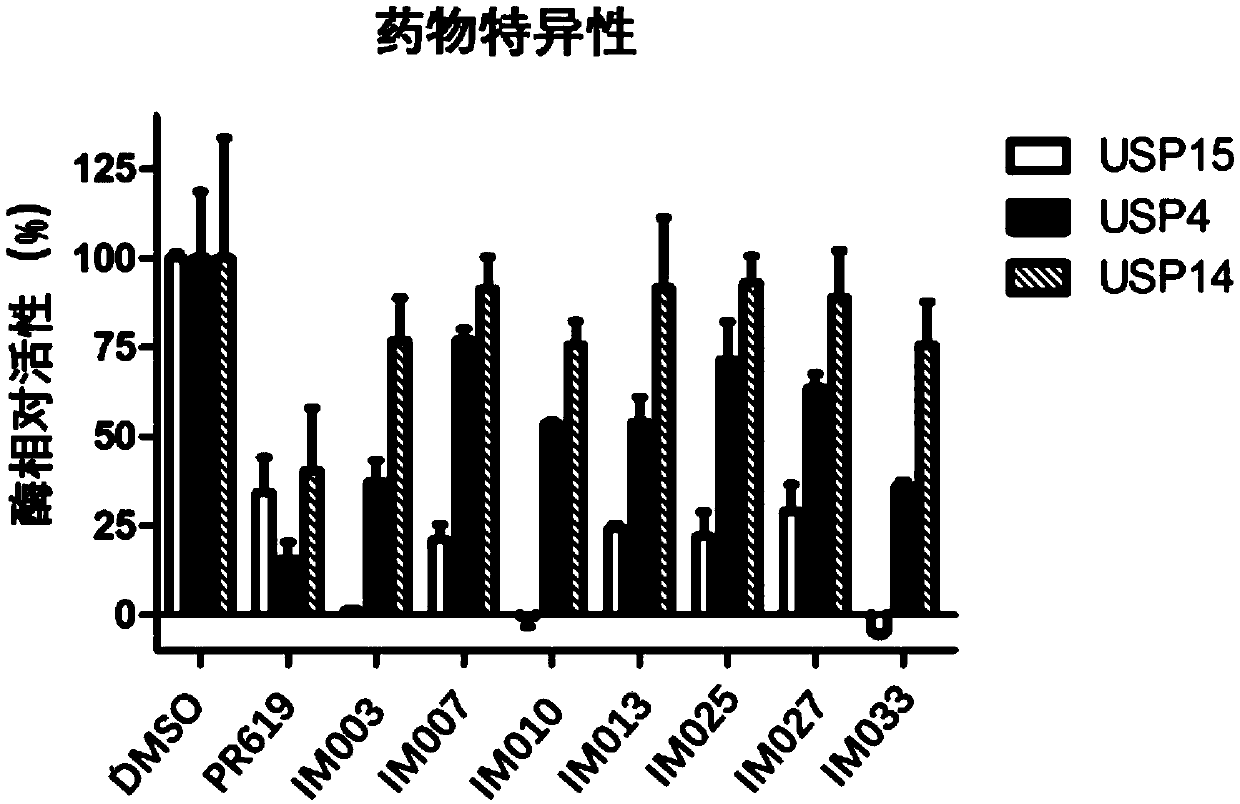
Image
Examples
Embodiment 1
[0096] Embodiment one: the preparation of each compound
Embodiment 1-1
[0097] Example 1-1, Synthesis of 6-(2-hydroxyethylamine) carbonyl-N-phenylacetyl-1,3,4,9-tetrahydro-9H-β-carboline (IM001)
[0098]
[0099] Take 4-hydrazinobenzoic acid methyl ester hydrochloride (405mg, 2mmol) and 4-chlorobutyraldehyde dimethyl acetal (367mg, 2.4mmol) and dissolve in 18mL ethanol-water mixed solvent (V / V, 5 / 1) After about 18 hours of reaction, the solution turned reddish brown, and the solvent was distilled off under reduced pressure. After evaporating to dryness, dissolve it with methanol (25mL), heat and stir, and when the temperature is 80°C, add SOCl dropwise 2 , then continued to heat and reflux at 80° C. for 1-2 hours, evaporated to dryness, and purified by column chromatography to obtain 6-methoxycarbonyltryptamine (205 mg, yield: 47%);
[0100] Dissolve 6-methoxycarbonyltryptamine (205 mg, 0.94 mmol) in glacial acetic acid (5 mL), add formaldehyde solution (71 μL, 0.94 mmol) dropwise under nitrogen protection, react at room temperature overnight,...
Embodiment 1-1 to 1-35
[0104] The preparation method of table 1. embodiment 1-1 to 1-35 compound IM-001 to IM-035
[0105]
[0106]
[0107]
[0108]
[0109]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com