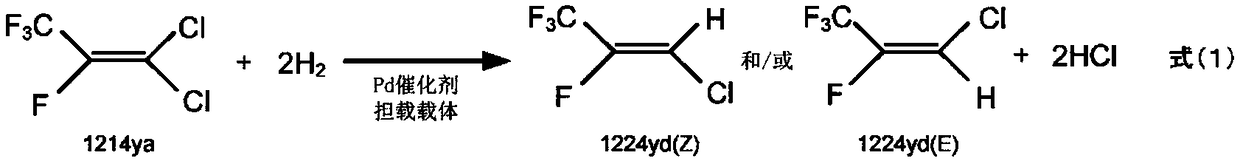
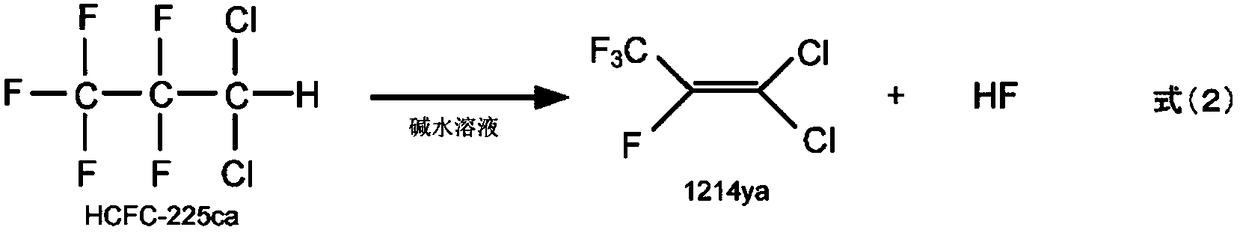
1-chloro-2,3,3,3-tetrafluoropropene manufacturing method
A manufacturing method, the technology of tetrafluoropropene, applied in the direction of organic chemical methods, chemical instruments and methods, dehalogenation preparation, etc., can solve the problems of unrecorded methods, and achieve the effect of easy implementation and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
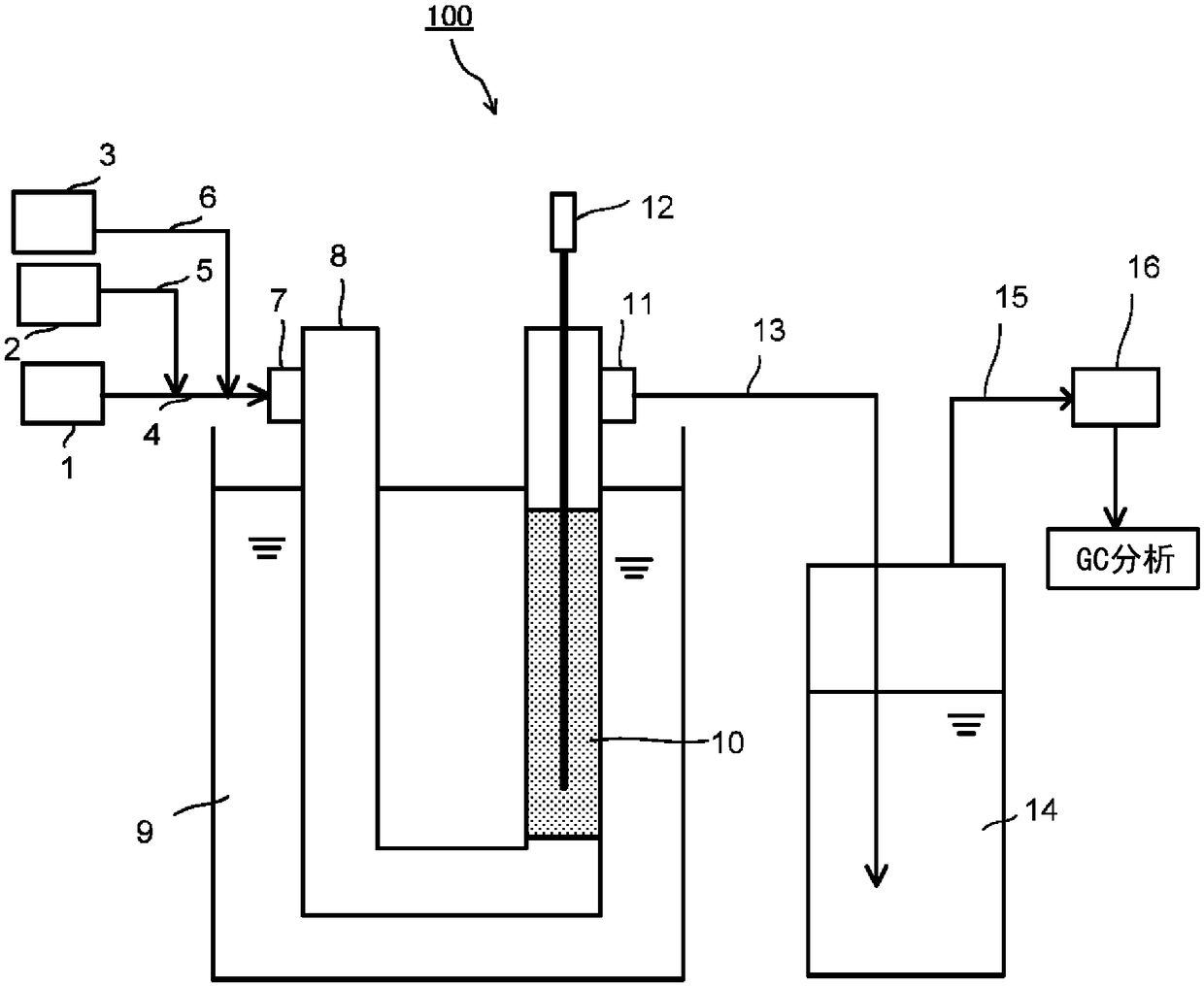
[0095] The present invention will be described in detail below by showing Examples and Comparative Examples. However, the present invention is not limited to the following description. Examples 1-4 are examples, and Examples 5-8 are comparative examples.
[0096] First, the palladium catalyst-supporting carrier used in each example was prepared as follows. Palladium catalyst-carrying carriers (X1) to (X3) are palladium catalyst-carrying carriers of the present invention, and palladium catalyst-carrying carriers (Cf1) to (Cf3) are palladium catalyst-carrying carriers for comparative examples. In addition, in the preparation of each palladium catalyst supporting carrier, palladium-supported activated carbon (N.E. Chemkit Co., Ltd.); hereinafter referred to as "palladium-supported activated carbon (A)"). The specific surface area of the palladium catalyst (palladium simple substance) supported in the palladium-supported activated carbon (A) is 198m as measured by the above-m...
preparation example 1
[0098] Palladium-supported activated carbon (A) was heat-treated at a temperature of 750°C for 10 hours in nitrogen to obtain a specific surface area of supported palladium of 6 m 2 / g of the palladium catalyst carrier (X1).
preparation example 2
[0100] Except that the heat treatment temperature in Preparation Example 1 was changed to 600°C, the specific surface area of supported palladium was 20m2 in the same manner as in Preparation Example 1. 2 / g of the palladium catalyst carrier (X2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com