Preparation method and application of CAR (Chimeric Antigen Receptor)-T cells of targeting HER2 (Human Epidermal Growth factor receptor 2)
A cellular and targeted technology, applied in the fields of immunology and immunotherapy, which can solve the problems of low HDR efficiency and imprecise gene editing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
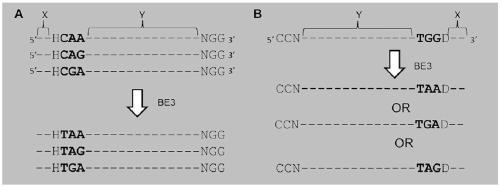
[0128] Embodiment 1 constructs BE3 plasmid
[0129] The BE3 plasmid rAPOBEC1-SpCas9-NLS-UGI-NLS was formed by fusing Cas9nickase with cytidine deaminase (APOBEC1), the structure of which is as follows figure 2 shown.
Embodiment 2
[0130] Example 2 Designing sgRNA bases
[0131] For 6 target genes: human PD1, LAG3, TIGIT, VISTA, 2B4 and CD160, this example selects the following target gene sequences to design the corresponding sgRNA, where the bold underline indicates PAM, and the italic underline indicates the candidate mutation code son:
[0132] The sgRNA against human PD1 (hPD-1) is:
[0133]
[0134] The sgRNA targeting human LAG3 (hLAG3) is:
[0135]
[0136] The sgRNA against human TIGIT (hTIGIT) is:
[0137]
[0138] The sgRNA against human VISTA (hVISTA) is:
[0139]
[0140] The human 2B4 sgRNA is:
[0141]
[0142] The sgRNA against human CD160 (hCD160) is:
[0143]
Embodiment 3
[0144] Electric transgene knockout of embodiment 3T cells
[0145] BE3-mediated base editing is performed on primary cells, and stop codons are introduced to achieve gene knockout. This example is the electrotransgenic knockout of primary cells on human T cells:
[0146] (1) Separation and purification of PBMC cells
[0147] A. Use an anticoagulant tube to collect peripheral blood, and shake it while collecting to fully mix the peripheral blood with the anticoagulant;
[0148] B. Mix equal volumes of peripheral blood cells and lymphocyte separation medium, centrifuge, and absorb buffy coat cells after centrifugation;
[0149] C. Centrifuge after mixing the obtained buffy coat cells with PBS or serum-free cell culture medium, and the precipitate is the crude product of the PBMC cells;
[0150] D. Repeat the above steps A-C three times for the crude PBMC cells obtained in step C to obtain isolated and purified PBMC cells.
[0151] (2) Enrichment of CD3 positive T cells
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com