A kind of aryl bithiazole compound and application
A bithiazole and compound technology, applied in the field of aryl bithiazole compounds, can solve problems such as no disclosure, no disclosure of compound activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
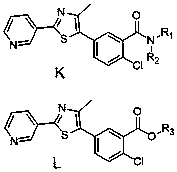
[0067] Example 1 Preparation of 2-chloro-N-methyl-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzamide as shown in compound number K1:
[0068] Add 2-chloro-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzoyl chloride (0.1g, 2.9mmol ), add 10mL tetrahydrofuran to dissolve, then add 33% methylamine aqueous solution (0.09g, 2.9mmol) and triethylamine (0.39g, 5.8mmol), and stir at room temperature for 2h. TLC detects the progress of the reaction, after the reaction is finished, filter, take the filtrate for precipitation, and recrystallize in petroleum ether to obtain the target compound shown in formula (K1);
[0069]
Embodiment 2
[0070] Example 2 Preparation of 2-chloro-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzamide as shown in compound number K2:
[0071] Add 2-chloro-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzoyl chloride (0.1g, 2.9mmol ), add 10mL tetrahydrofuran to dissolve, then add ammonia water 0.1g (the molar weight of ammonia is 2.9mmol) and triethylamine (0.39g, 5.8mmol), and stir at room temperature for 2h. TLC detects the progress of the reaction, after the reaction is finished, filter, take the filtrate for precipitation, and recrystallize in petroleum ether to obtain the target compound shown in formula (K2);
[0072]
Embodiment 3
[0073] Example 3 Preparation of methyl 2-chloro-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzoate as shown in compound number L49:
[0074]Add 2-chloro-5-(4-methyl-2-(pyridin-3-yl)thiazol-5-yl)benzoyl chloride (0.1g, 2.9mmol ), add 10mL tetrahydrofuran to dissolve, then add methanol (0.09g, 2.9mmol) and triethylamine (0.39g, 5.8mmol) and stir at room temperature for 2h. TLC detects the progress of the reaction, after the reaction is finished, filter, get the filtrate for precipitation, and recrystallize in petroleum ether to obtain the target compound shown in formula (L49);
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com