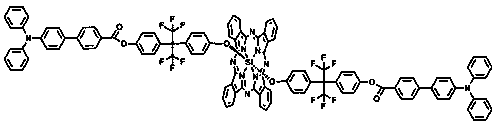
Trianilino fluoroaryl benzyl ether dendrimer substituted silicon phthalocyanine and its preparation method and application
A technology of triphenylamine-based aryl benzyl ether and fluoroaryl benzyl ether, which is applied in the field of complexes to achieve the effects of strong resistance to temperature and oxidation, inhibition of self-aggregation behavior, and strong solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Dichlorosilicon phthalocyanine (SiPcCl 2 )Synthesis
[0027] Add 1,3-diiminoisoindoline (7.28 g, 50.15 mmol), silicon tetrachloride (8.3 mL) and quinoline (83 mL) respectively into a three-necked flask, stir and reflux for 30 min at 220 °C , cooled to room temperature, poured into 500mL methanol solution, stirred and stood for about 1h, filtered, and the filter residue was washed with 35 mL each of acetone, methanol, dichloromethane, methanol and other solvents, and after drying, 3.6759 g of purple solid was obtained. The yield was 48.62%.
[0028] ) Synthesis of 4'-(diphenylamino)-[1,1'-biphenyl]-4-carboxylic acid (abbreviated as TPA-COOH in the present invention)
[0029] In a three-neck round bottom flask (250mL), add 4-bromotriphenylamine (1.260 g, 3.89 mmol), 4-carboxyphenylboronic acid (0.64 g, 3.86 mmol), tetrakis(triphenylphosphine) palladium Pd(PPh 3 ) 4 (0.140 g, 0.120 mmol), after anhydrous and anaerobic operation, inject ethylene glycol dimethyl ethe...
Embodiment 2
[0039] The specific steps are the same as in Example 1: in process 2), 4-bromotriphenylamine was changed to (2.52 g, 7.78 mmol), 4-carboxyphenylboronic acid was changed to (1.28 g, 7.72 mmol), and the reaction temperature was changed to 90°C; The time was changed to 36 h. Other reaction conditions were the same, and 1.574 g of tan powder was obtained with a yield of 55.8%.
[0040] The specific steps are the same as in Example 1: Process 3) TPA-COOH is changed to (0.36 g, 1.00 mmol), fluorobisphenol A is changed to (0.504 g, 1.50 mmol), DMAP is changed to (36 mg, 0.3 mmol), EDC HCl was changed to (0.25 g, 1.30 mmol), the reaction time was changed to two nights, and other reaction conditions were the same, and 0.40 g of yellow powder was obtained with a yield of 58.8%.
[0041] The specific steps are the same as in Example 1: Process 4) TPA-COOH was changed to (0.36 g, 1.00 mmol), bisphenol A was changed to (0.342 g, 1.50 mmol), DMAP was changed to (36 mg, 0.3 mmol), EDC HCl w...
Embodiment 3
[0045] Evaluation of Photodynamic Activity of TPA-FBPA-SiPc on MCF-7 Breast Cancer Cells
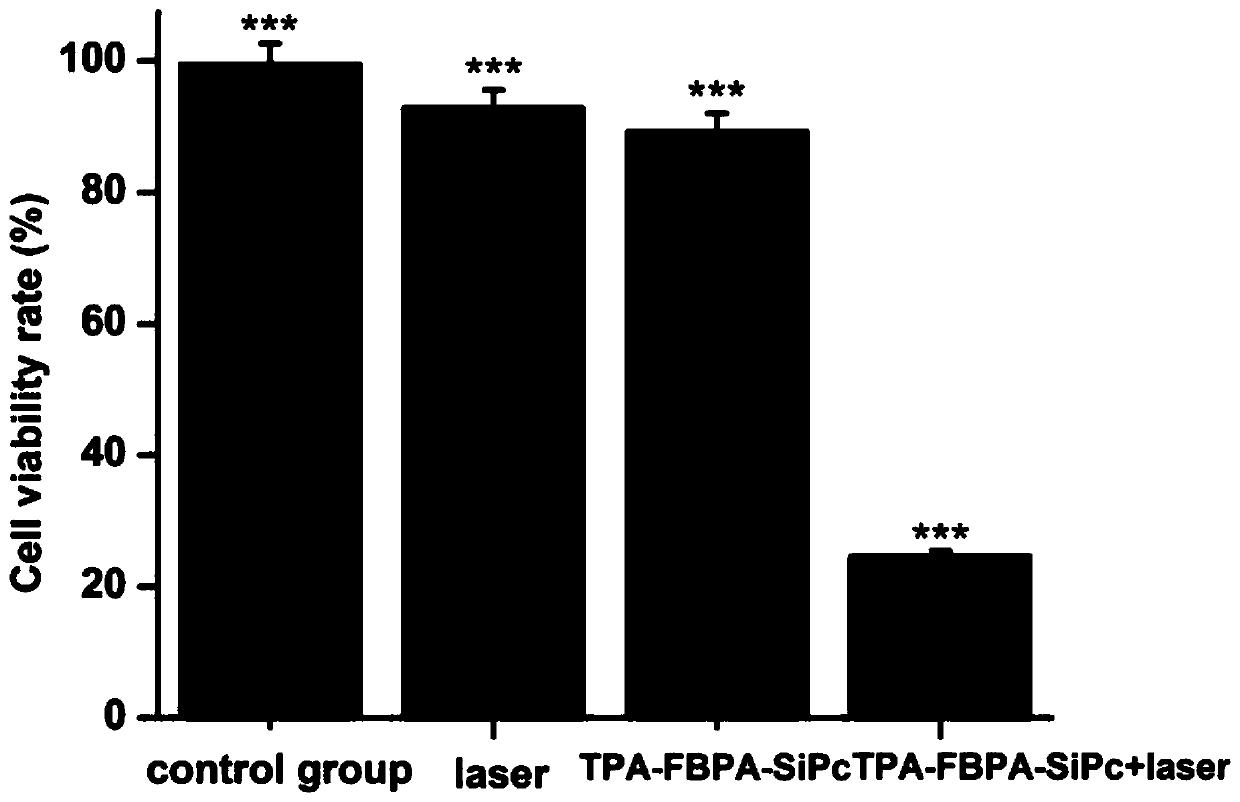
[0046] Inoculate a 96-well plate, 100 uL per well containing 5×10 3 After culturing for 24 hours, TPA-FBPA-SiPc was added to the culture medium. Without laser irradiation, the drug is protected from light for 24 hours. Cells cultured without adding nanoparticles, only adding blank solvent (normal saline) and without laser radiation were used as the negative control group. CCK-8 measures cell viability. See figure 1 , figure 1 Cell viability under different conditions: TPA-FBPA-SiPc(5μM), Laser (100 J / cm2)) (*p<0.05, ***p<0.001, statistical analysis compared with PBS.
[0047] From figure 1 It can be seen that DSPE-PEG 2000 @TPA-FBPA-SiPc nanoparticles have obvious inhibitory effect on breast cancer.
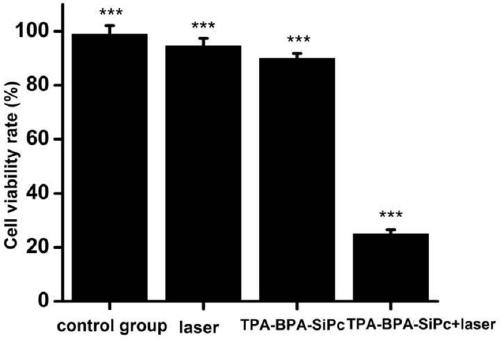
[0048] Photodynamic Activity Evaluation of MCF-7 Breast Cancer Cells
[0049] Inoculate a 96-well plate, 100 uL per well containing 5×10 3 After culturing for 24 hours, TPA-BPA-Si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com