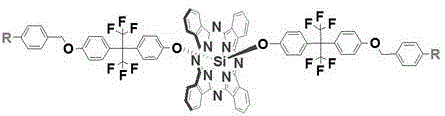
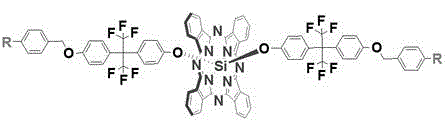
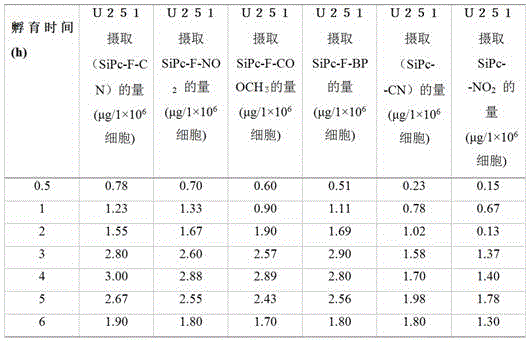
Fluorinated aryl benzyl ether dendritic phthalocyanine silicon complex and its preparation method and application
A technology of fluorinated aryl benzyl ether and substituted aryl benzyl ether, which is applied in the field of fluorinated aryl benzyl ether dendritic phthalocyanine silicon complex and its preparation, to achieve the effect of inhibiting self-aggregation behavior and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Dichlorosilicon phthalocyanine (SiPcCl 2 )Synthesis
[0030] Add 1,3-diiminoisoindoline (3.7g, 25.5mmol), silicon tetrachloride (4.2mL) and quinoline (42mL) into a three-necked flask respectively, stir and reflux at 220°C for 30min, and the mixture When the temperature was lowered to 80°C, it was poured into methanol (80 mL), filtered while it was hot, and the filter residue was washed with 35 mL each of toluene, quinoline, methanol and acetone. After drying, 2.2 g of a purple solid was obtained, with a yield of 60%.
[0031] 2) 2-(4-hydroxybenzene)-2'-(4-(4-cyano-benzyloxy)hexafluoropropane (abbreviation: G 1 -F-CN-OH) synthesis
[0032] Add p-cyanobenzyl bromide (2.4g, 12.0mmol), anhydrous potassium carbonate (2.0g, 14.5mmol), 2,2-bis(4-hydroxyphenyl)hexafluoropropane (4.9g, 14.4mmol), acetone (50mL), stirred vigorously at 55°C and refluxed for 48h, filtered, collected the filtrate, and evaporated the solvent under reduced pressure to obtain a white crude produc...
Embodiment 2
[0049] In Example 1, the process 2) was changed to 4.2g of p-cyanobenzyl bromide, 9.0g of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 50mL of acetone, 2h of reaction time, and other Under the same reaction conditions, 7.5 g of a white powdery solid substance was obtained, with a yield of 73%.
[0050] In embodiment one, process 3) SiPcCl 2 Change to 0.4g, G 1 -F-CN-OH was changed to 0.8g; the temperature was changed to 150°C and other reaction conditions were the same, the blue-green solid was 0.33g, and the yield was 70.0%.
[0051] In Example 1, the process 4) p-nitrobenzyl bromide was changed to 3.8g, 2,2-bis(4-hydroxyphenyl)hexafluoropropane was changed to 6.0g, acetone was changed to 60mL, the reaction time was changed to 3h, and other Under the same reaction conditions, 5.5 g of a white powdery solid substance was obtained, with a yield of 47%.
[0052] In embodiment one, process 5) SiPcCl 2 Change to 0.5g, G 1 -F-CN-OH was changed to 1.0g; the temperature was change...
Embodiment 3
[0056] In Example 1, the process 2) was changed to 3.2g of p-cyanobenzyl bromide, 6.3g of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 30mL of acetone, 3h of reaction time, and other Under the same reaction conditions, 4.7 g of a white powdery solid substance was obtained, with a yield of 46.0%.
[0057] In embodiment one, process 3) SiPcCl 2 Change to 0.3g, G 1 -F-CN-OH was changed to 0.6g; the temperature was changed to 140°C, and other reaction conditions were the same, the blue-green solid was 0.24g, and the yield was 60.0%.
[0058] In Example 1, the process 4) p-nitrobenzyl bromide was changed to 3.6g, 2,2-bis(4-hydroxyphenyl)hexafluoropropane was changed to 5.8g, acetone was changed to 50mL, the reaction time was changed to 4h, and other Under the same reaction conditions, 5.0 g of a white powdery solid substance was obtained, with a yield of 46.0%.
[0059] In embodiment one, process 5) SiPcCl 2 Change to 0.4g, G 1 -F-CN-OH was changed to 0.7g; the temperature was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com