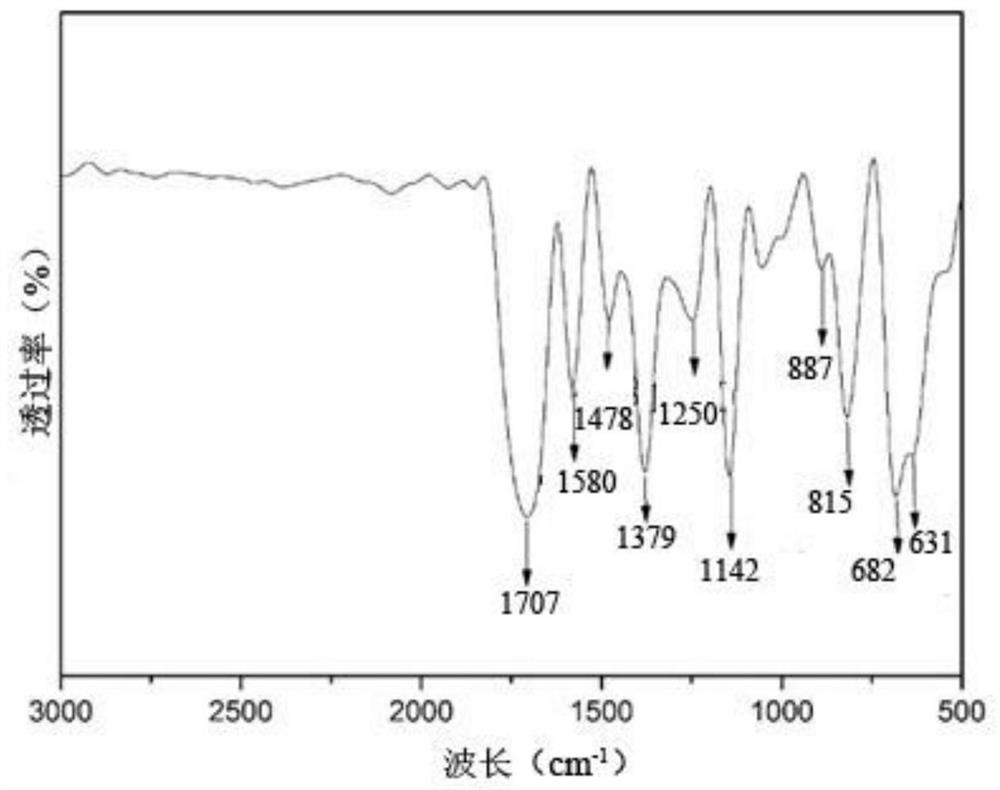
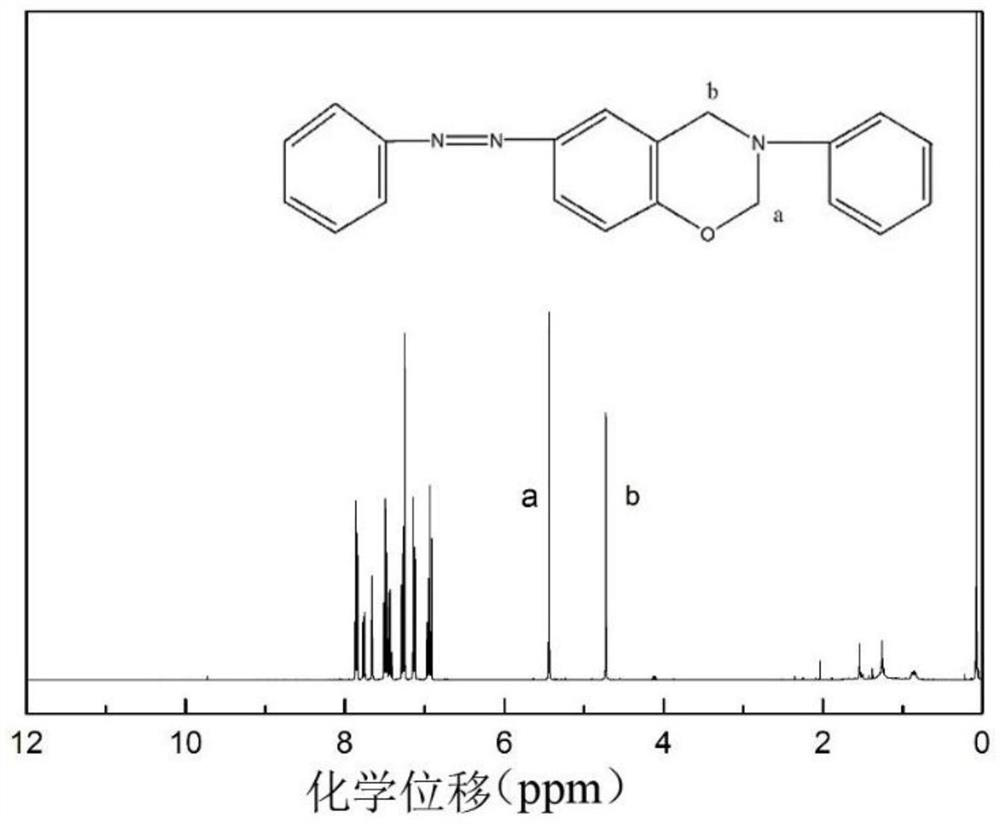
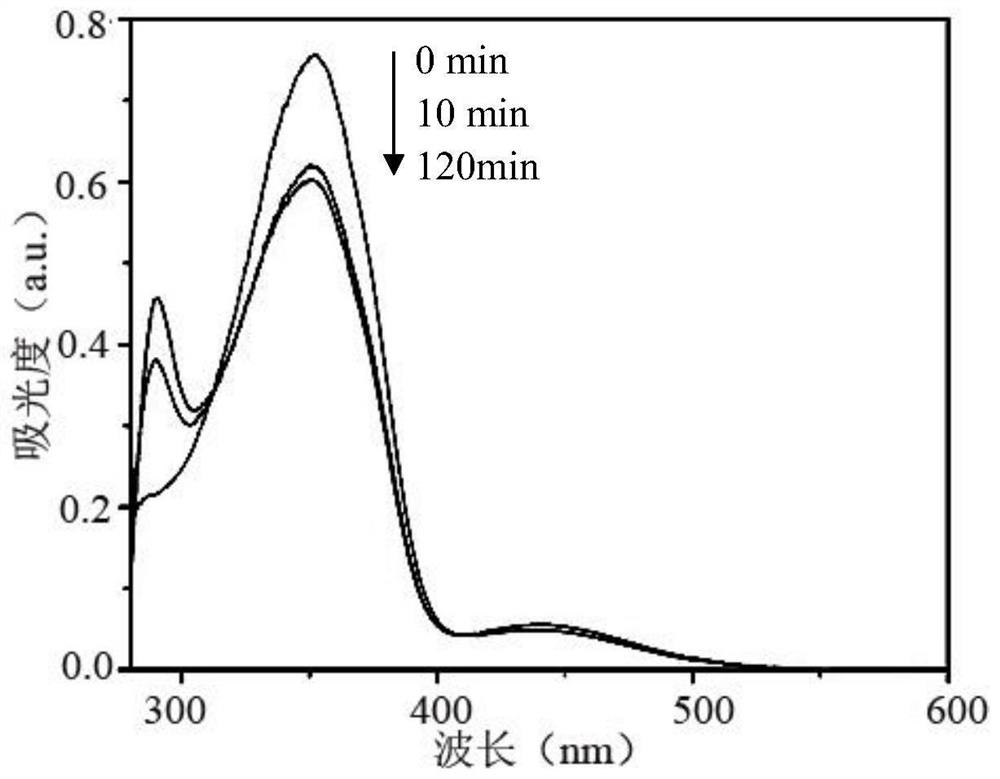
A kind of photoactive benzoxazine resin and preparation method thereof
A benzoxazine and photoactive technology, applied in the field of novel photoactive benzoxazine resin and its preparation, can solve the problems such as no photoactive benzoxazine products, achieve low softening temperature, simple curing process, Effect of low volume shrinkage of material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of photoactive benzoxazine resin, comprising the following steps;
[0036] (1) After adding 33 parts by weight of paraformaldehyde and 100 parts by weight of 4-(phenylazo)phenol into the reactor, add 1000 parts by weight of toluene, and mechanically stir at 400rpm at 25°C for 2h, then Raise the temperature of the reaction liquid system to 45°C; (2) slowly and gradually add 46 parts by weight of aniline to the above system, keep the temperature at 45°C, rotate at 400rpm, stir and react for 2h under nitrogen atmosphere; (3) raise the temperature of the reaction liquid system to 130°C, reflux reaction for 8 hours; (4) Cool the reaction system to room temperature, wash with alkali and water for 3 times, separate the organic phase, remove toluene by rotary evaporation, and obtain the photoactive benzo with azo structure after drying. Oxazine monomer; (5) heating the photoactive benzoxazine monomer to 180° C. for 2 hours for curing reaction, and then rais...
Embodiment 2
[0046] A preparation method of photoactive benzoxazine resin, comprising the following steps;
[0047] (1) After adding 34 parts by weight of paraformaldehyde and 100 parts by weight of phenol into the reactor, add 1200 parts by weight of tetrahydrofuran, and mechanically stir at 350 rpm for 1 hour at 30°C, then raise the temperature of the system to 50°C; (2) Slowly and gradually add 100 parts by weight of 4-aminoazobenzene to the above system, keep the constant temperature at 50°C, rotate at 350rpm, stir and react at constant temperature under nitrogen atmosphere for 1h; (3) raise the temperature of the reaction solution to 75°C, and reflux for 6h (4) the system is cooled to room temperature, after washing with alkali and water for 4 times, the organic phase is separated, the toluene is removed by rotary evaporation, and after drying, the photoactive benzoxazine monomer containing an azo structure is obtained; (5) The temperature of the photoactive benzoxazine monomer contai...
Embodiment 3
[0050] A preparation method of photoactive benzoxazine resin, comprising the following steps;
[0051] (1) After adding 60 parts by weight of paraformaldehyde and 205 parts by weight of 2-phenylazo-4 methylphenol into the reactor, add 1500 parts by weight of dioxane, and mechanically After stirring for 3 hours, raise the temperature of the system to 65°C; (2) slowly and gradually add 105 parts by weight of 4,4'-azodiphenylamine to the above system at a constant temperature of 65°C and a speed of 300rpm, under a nitrogen atmosphere at a constant temperature and constant speed React for 4 hours; (3) raise the temperature of the reaction solution to 110° C., and reflux for 7 hours; (4) cool the system to room temperature, wash with alkali and water for 6 times, separate the organic phase, and remove dioxane by rotary evaporation. After drying, a photoactive benzoxazine monomer containing an azo structure is obtained; (5) the photoactive benzoxazine monomer containing an azo struc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com