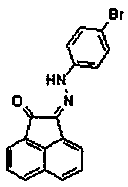
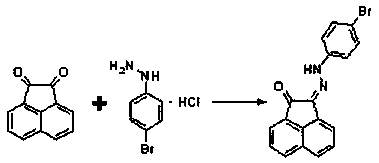
Compound (2z)-2-((4-bromophenyl)hydrazono)acenaphthyl-1-one and its preparation method and application
A technology of a compound, bromophenyl, applied in the field of compound (2Z)-2-((4-bromophenyl)hydrazono)acenaphthyl-1-one and its preparation and application, which can solve the problem of low product yield, Complex reaction conditions, high cost of raw materials, etc., to achieve the effect of safe and reliable operation, mild reaction temperature, easy control, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 100mL round-bottomed flask, add 1.82g (10.0mmol) acenaphthenequinone, 2.57g (11.5mmol) p-bromophenylhydrazine hydrochloride and 1g anhydrous sodium sulfate in sequence, 50mL ethanol as a solvent, nitrogen protection, and magnetic stirring Heat to about the boiling point of ethanol, reflux for 5 hours, cool to room temperature, filter with suction, wash the filter cake with an appropriate amount of water, and drain to obtain an orange-yellow solid, which is recrystallized to obtain 1.78 g of orange-yellow crystals. Yield 50.6%. 1H-NMR (400MHz, CDCl3, 25°C, TMS): δ=12.56(s, 1H); 8.12(d, J=8.0Hz, 1H); 8.01(d, J=8.0Hz, 1H); 7.85( d,J=8.0Hz,1H);7.80(d,J=8.0Hz,1H);7.73(t,J=8.0Hz,1H);7.66(t,J=8.0Hz,1H);7.47(d, J=8.8Hz,2H);7.29(d,J=8.8Hz,2H).MS(ESI):m / z=350.01,352.00,351.01,353.01(M+;Abundance ratio=5:5:1:1); calcdforC18H11BrN2O[M+Na]+:372.84,374.85,373.84,375.84(Abundance ratio=5:5:1:1).Elem.Anal.Calcd.forC18H11BrN2O(351.2):C,61.56;H,3.16;Br,22.75; N,7.98;O,4.56.Found:...
Embodiment 81
[0042] In a 100mL round bottom flask, add 1.82g (10.0mmol) of acenaphthenequinone, 1.14g (5.1mmol) of p-bromophenylhydrazine hydrochloride and 1g of anhydrous potassium sulfate, 50mL of ethanol as a solvent, nitrogen protection, and heating under magnetic stirring To the boiling point of ethanol, reflux for about 12 hours, cool to room temperature, filter with suction, wash the filter cake with an appropriate amount of water, and drain to obtain an orange-yellow solid, which is recrystallized to obtain 1.66 g of orange-yellow crystals. Yield 92.6%.
[0043] Examples 82–96:
[0044] The reaction solvent, reaction time and other operations were the same as in Example 81, but the molar ratio of the reactants was changed, and the quality and yield of the final product are shown in Table 6 below.
[0045] Table 6 ethanol as solvent, product quality and its productive rate under the situation of different molar ratios of reactant
[0046] Example
82
83
84
...
Embodiment 161
[0064] In a 100mL round-bottomed flask, add 1.82g (10.0mmol) acenaphthenequinone, 2.57g (11.5mmol) p-bromophenylhydrazine hydrochloride and 1g anhydrous sodium sulfate in sequence, 50mL ethanol as a solvent, nitrogen protection, and magnetic stirring Heated to about 60°C, refluxed for 12 hours, cooled to room temperature, filtered with suction, washed the filter cake with an appropriate amount of water, and dried to obtain an orange-yellow solid, which was recrystallized to obtain 2.27 g of orange-yellow crystals. Yield 64.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com