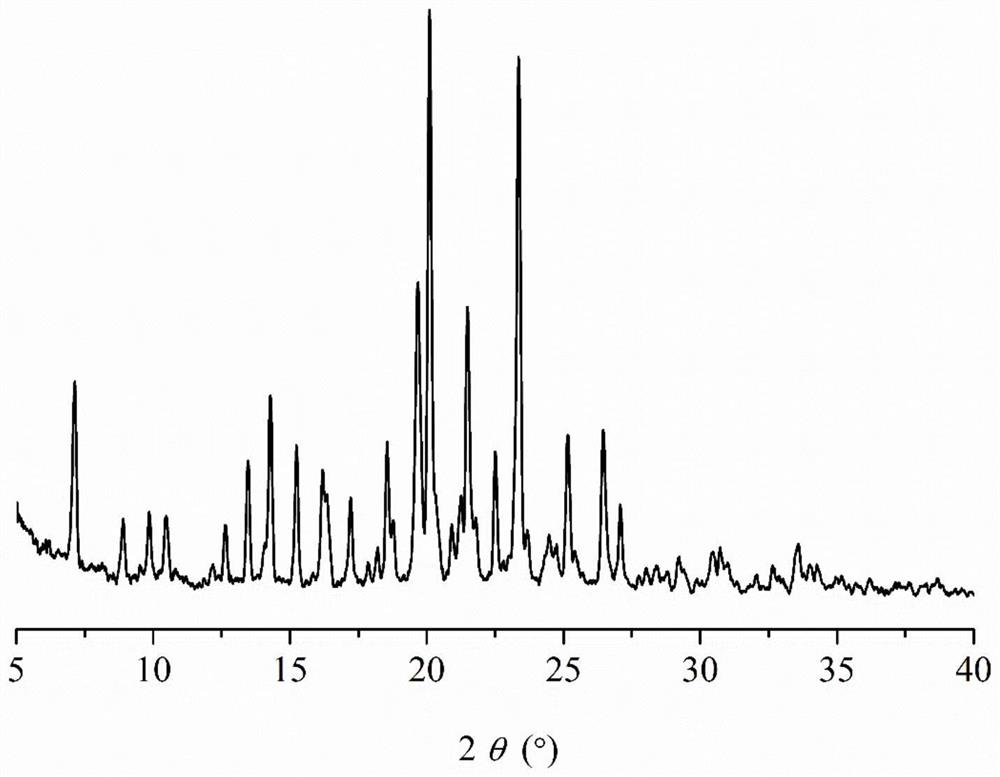
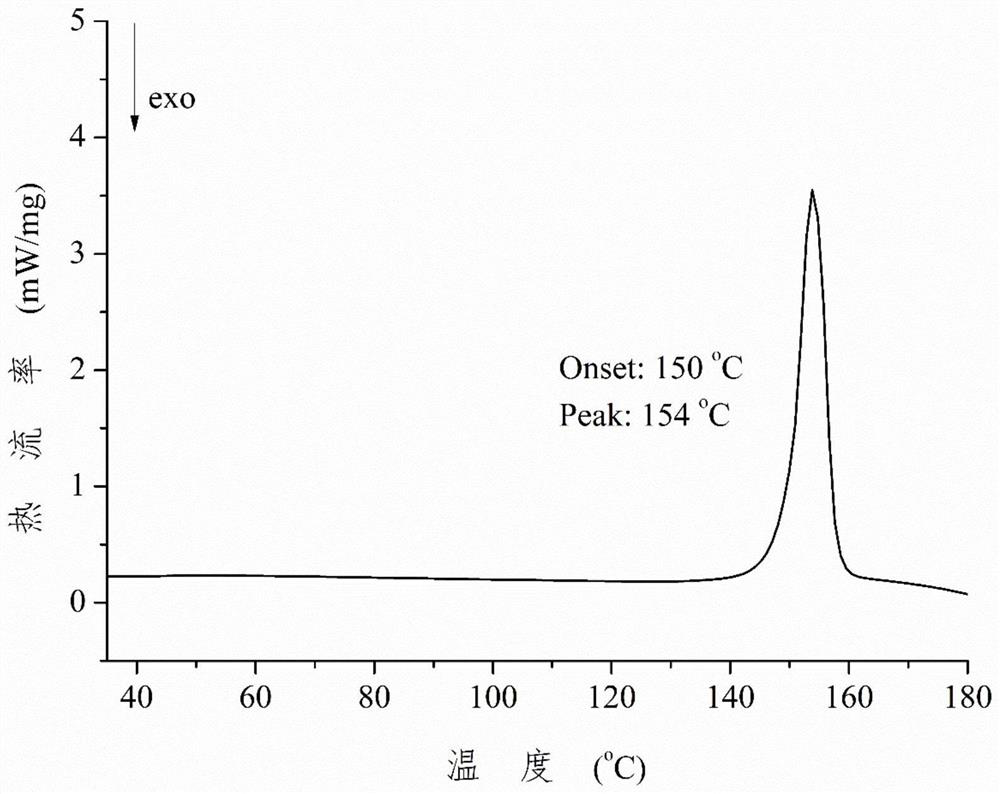
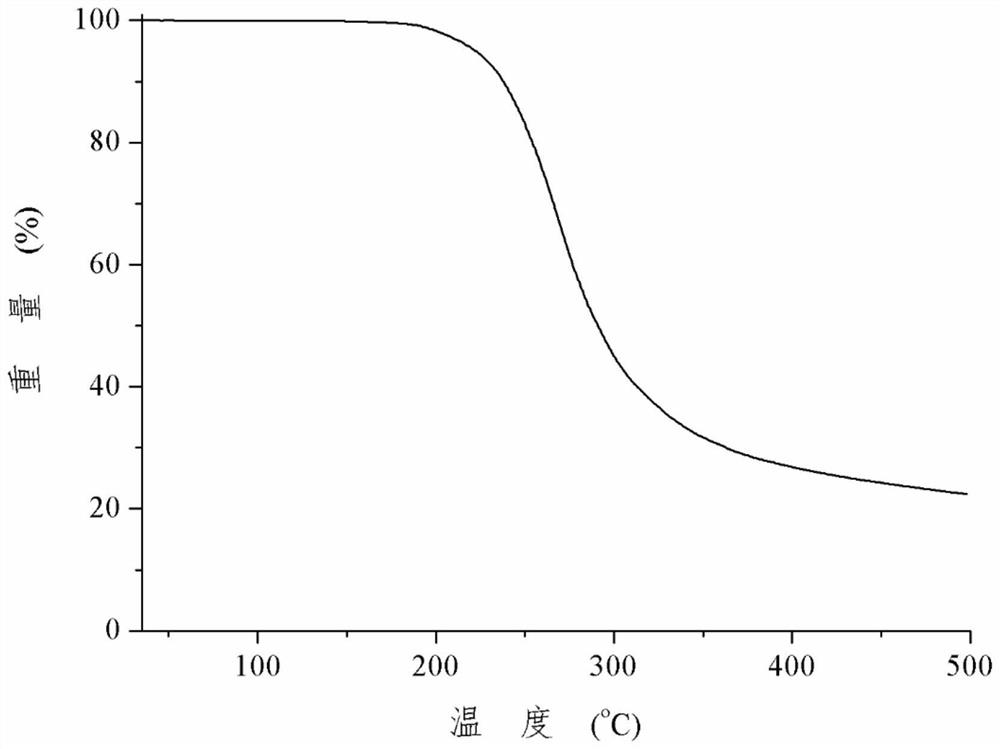
A kind of rosiglitazone gentisate and preparation method thereof
A technology of rosiglitazone gentisate and gentisic acid, which is applied in the field of chemical medicine, can solve the problems of high price, limited clinical application, etc., and achieves the advantages of good reproducibility, large apparent solubility and easy control of crystallization process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of rosiglitazone gentisate is to react rosiglitazone and gentisic acid in a solvent, and crystallize by stirring, ultrasonic, volatilization or cooling to obtain rosiglitazone gentisate.
[0038] Preferably, in the preparation method, the volume ratio of the sum of the mass of rosiglitazone and gentisic acid to the solvent is (50-110) mg:1 mL.
[0039] Preferably, in the preparation method, the molar ratio of rosiglitazone and gentisic acid is 1:(0.9-2).
[0040] Preferably, in the preparation method, the solvent is at least one of alcohol-based solvents, ester-based solvents, ketone-based solvents, ether-based solvents, and nitrile-based solvents.
[0041] Further, alcoholic solvents include but are not limited to methanol, ethanol, and isopropanol.
[0042] Further, ester solvents include but are not limited to methyl acetate, ethyl acetate, isopropyl acetate, ethyl formate,
[0043] Further, ketone solvents include but are not limited to acet...
Embodiment 1
[0053] Weigh 1.43 g of rosiglitazone and 0.61 g of gentisic acid, add them to 20 mL of ethyl formate to obtain a suspension, stir the suspension at room temperature for 48 h, filter, and dry the obtained white solid at 70 ° C for 48 h to obtain rosiglitazone. Solid sample of glitazone gentisate.
Embodiment 2
[0055] 35.8 mg of rosiglitazone and 15.8 mg of gentisic acid were weighed, added to 1 mL of ethyl formate to obtain a suspension, the suspension was stirred at room temperature for 24 h, filtered, and the obtained white solid was dried to obtain rosiglitazone dragon Solid sample of cholate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com