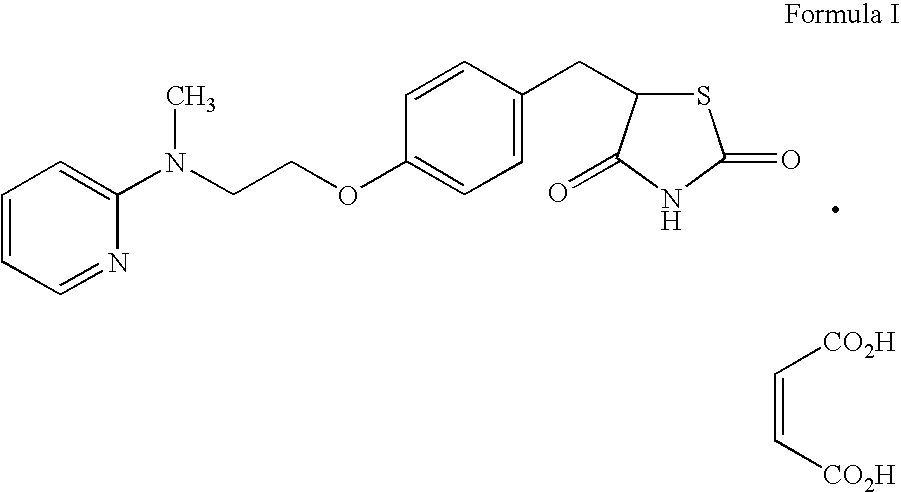
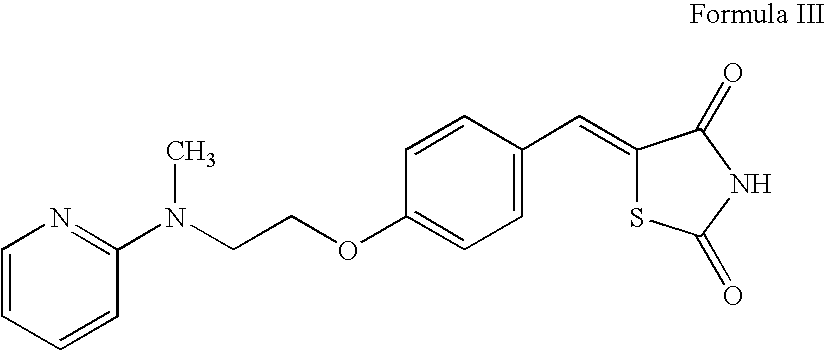
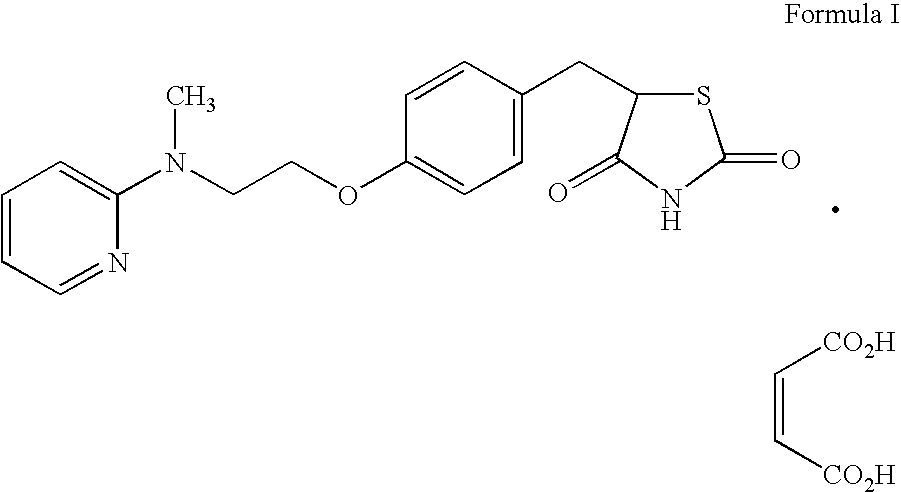
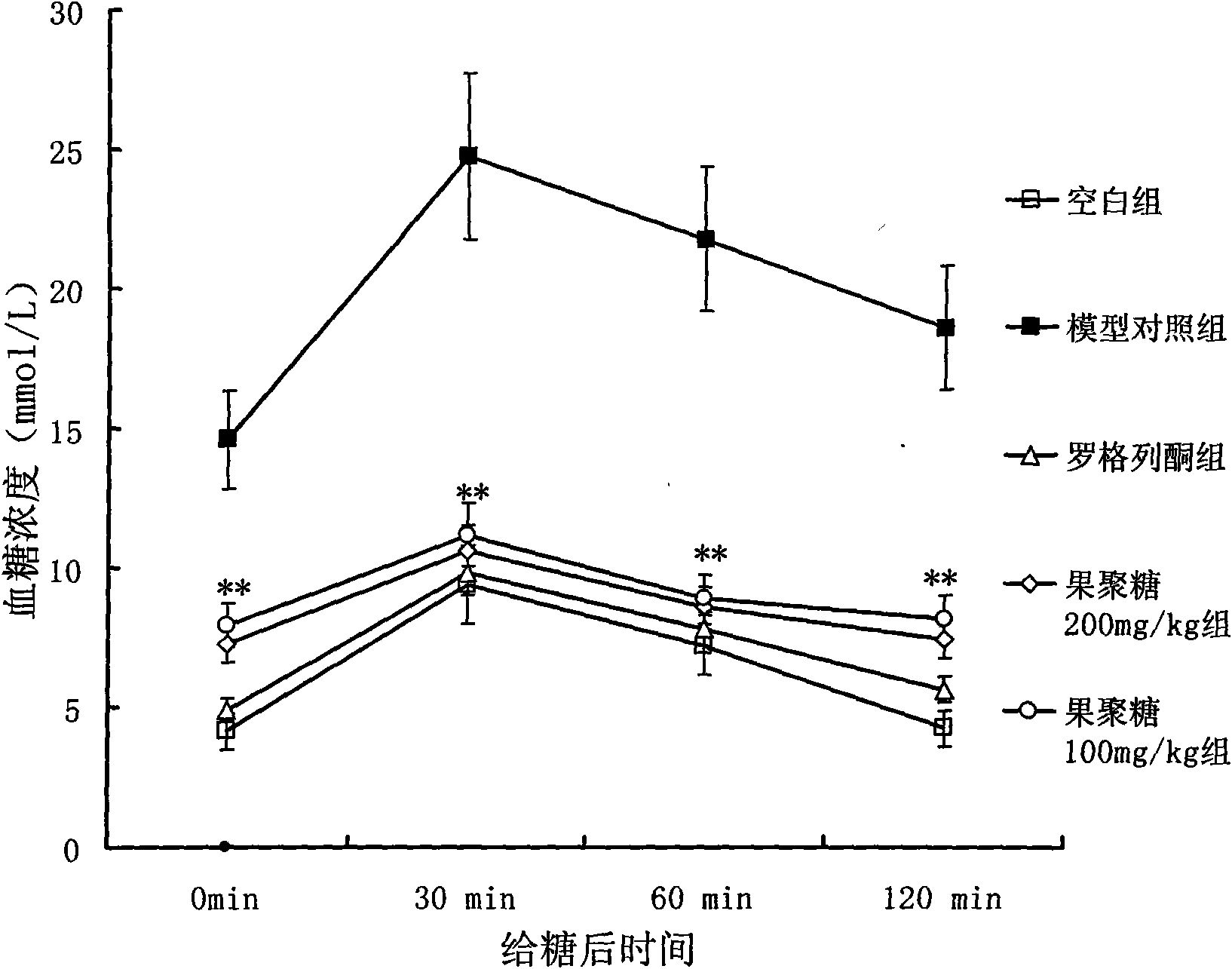
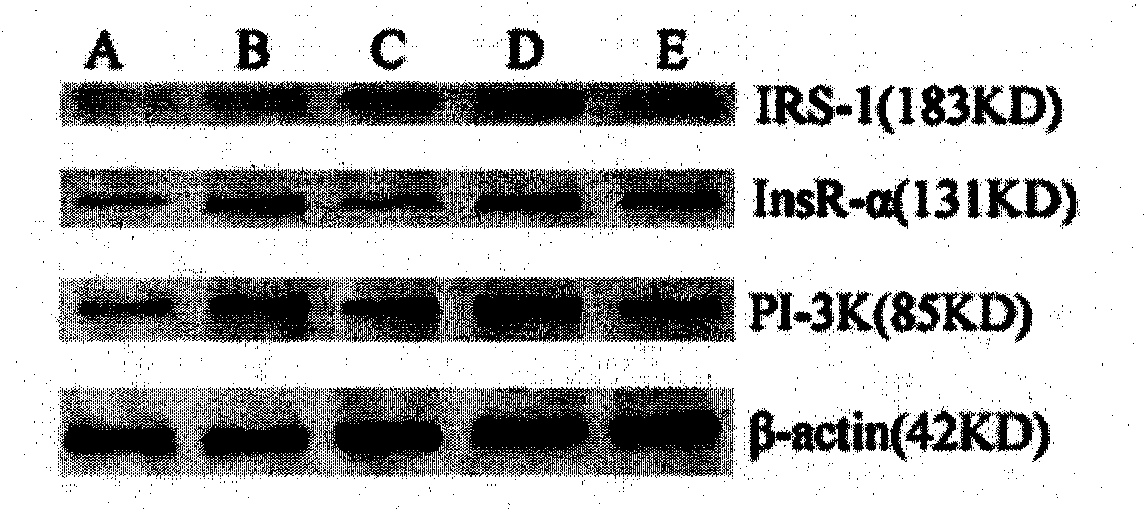
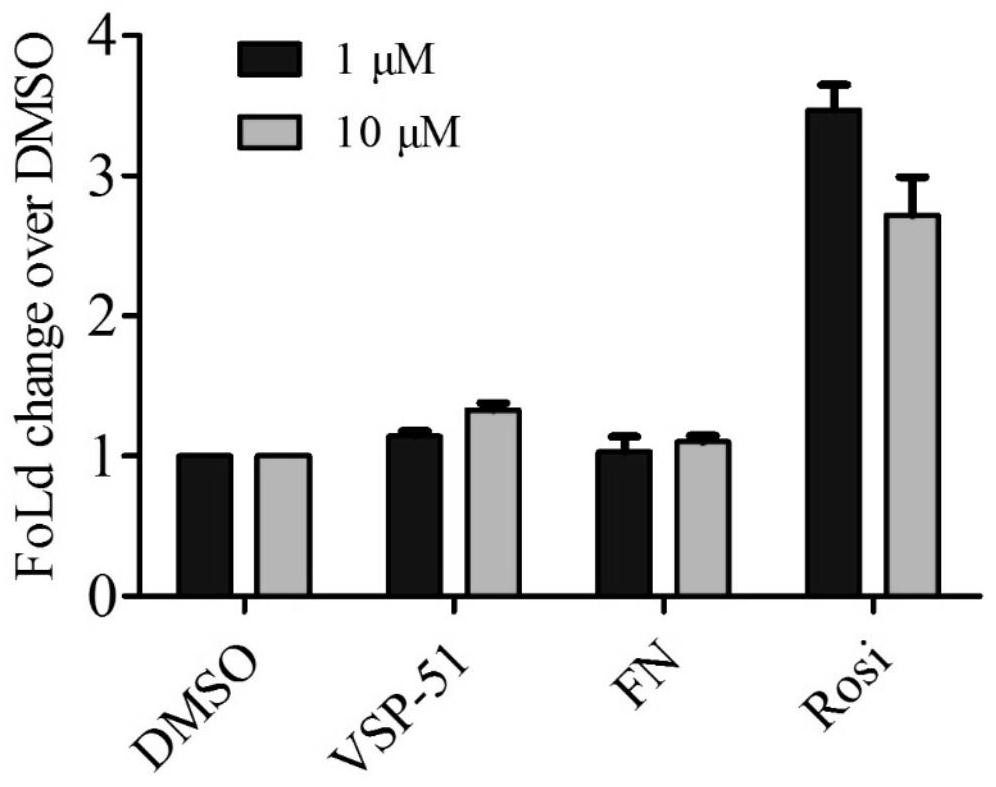
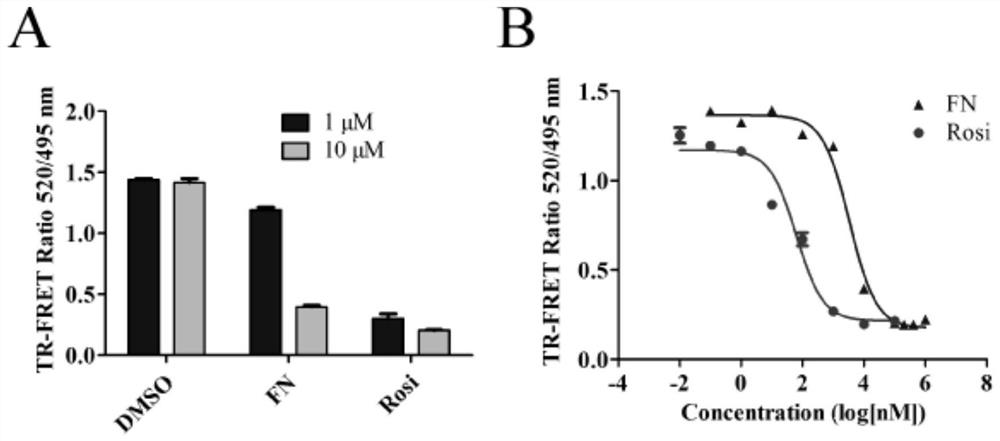
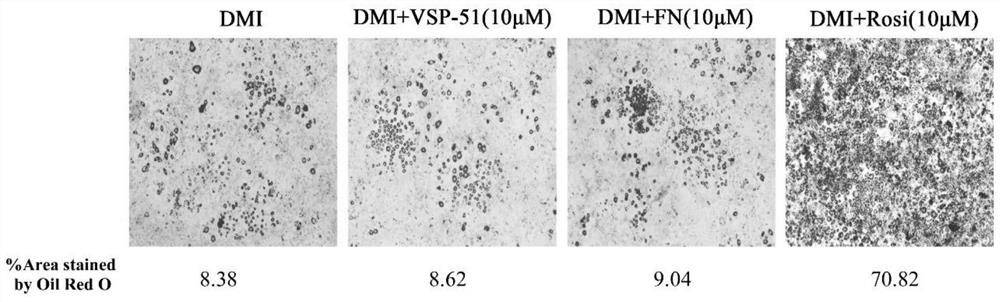
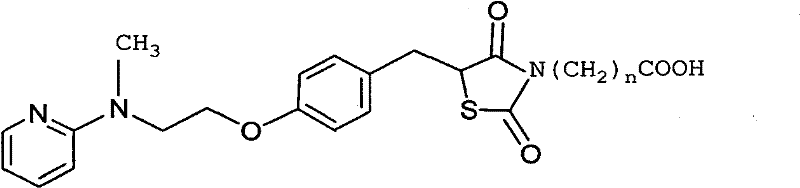
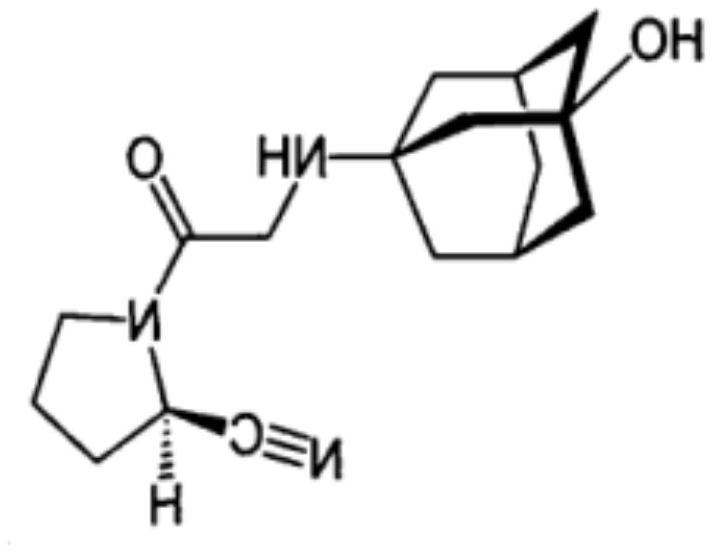
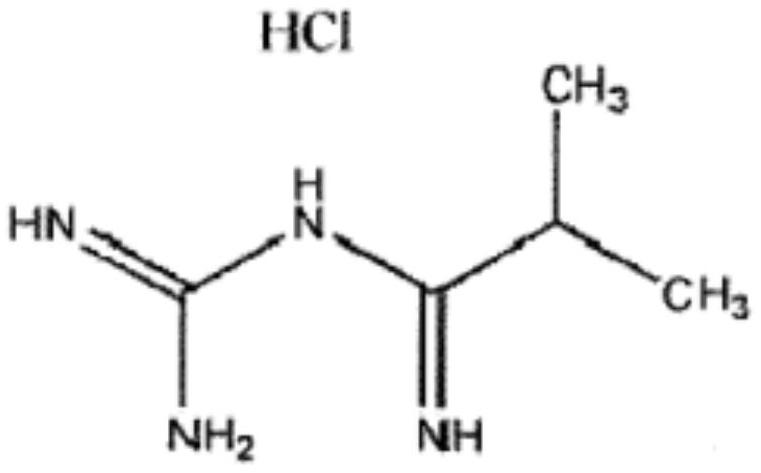
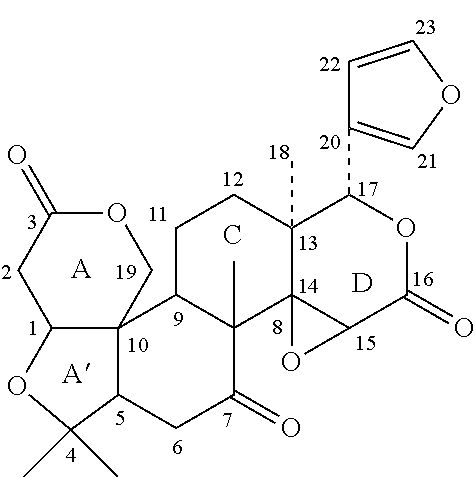
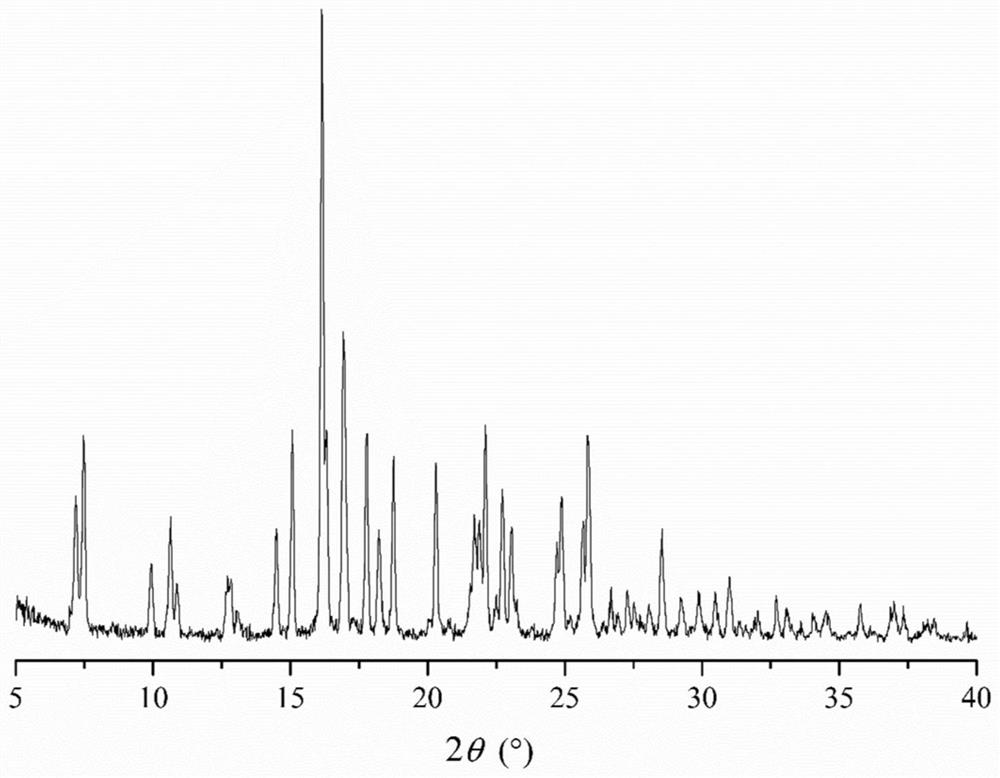
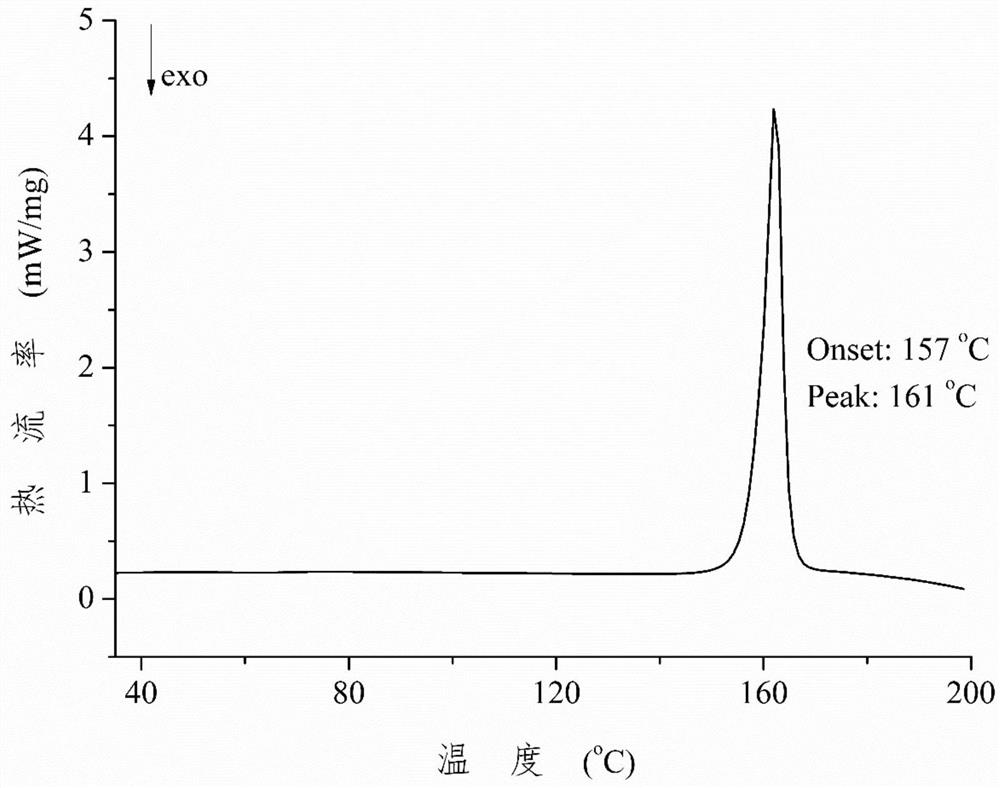
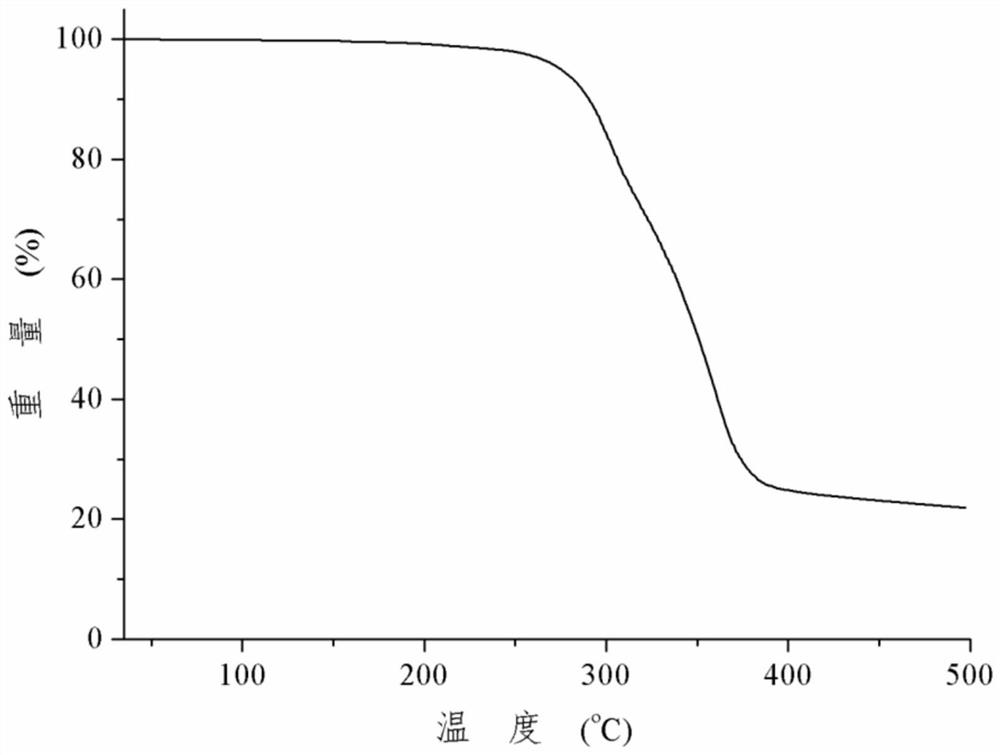
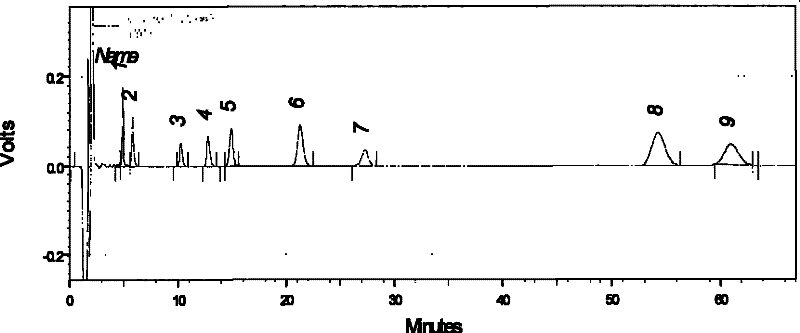
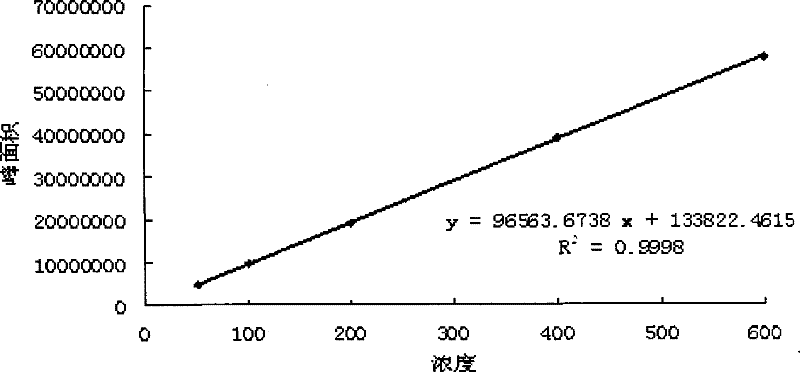
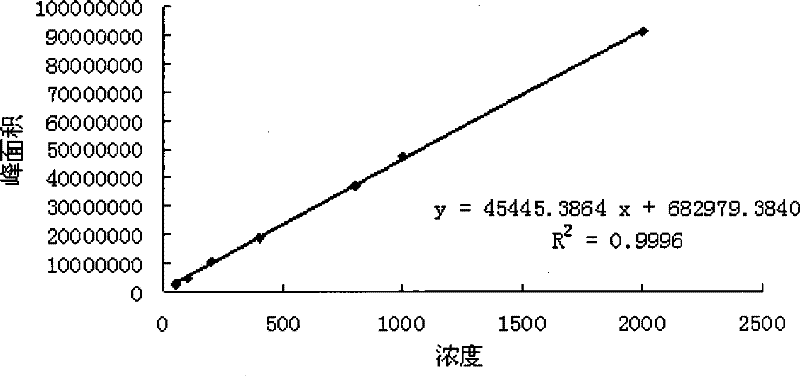
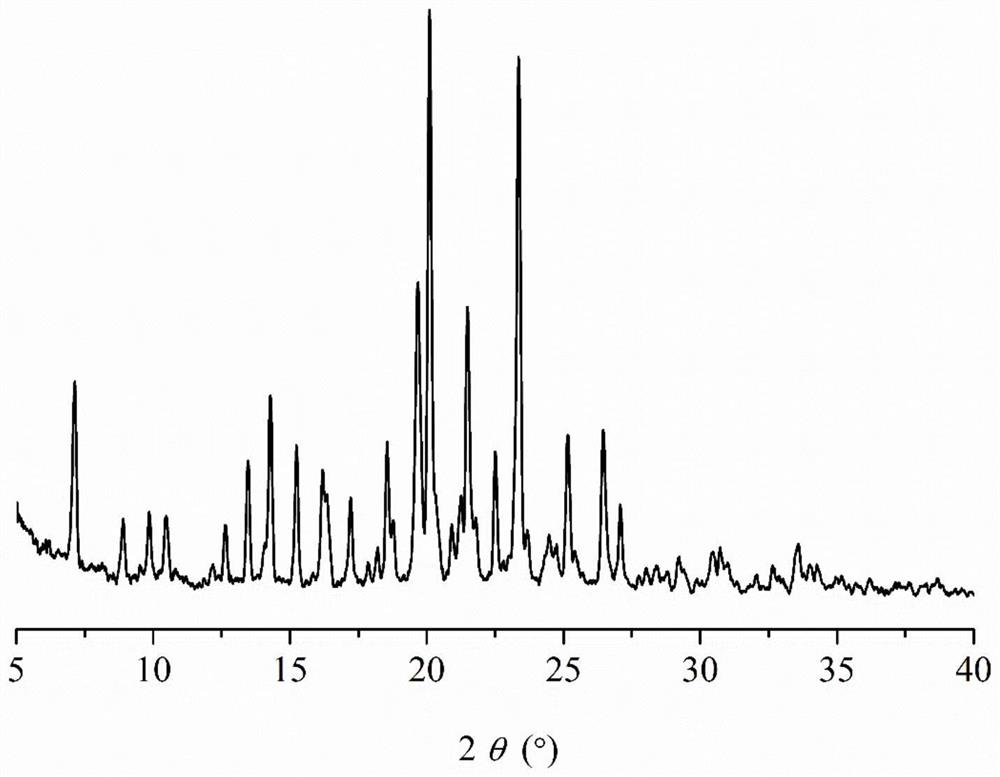
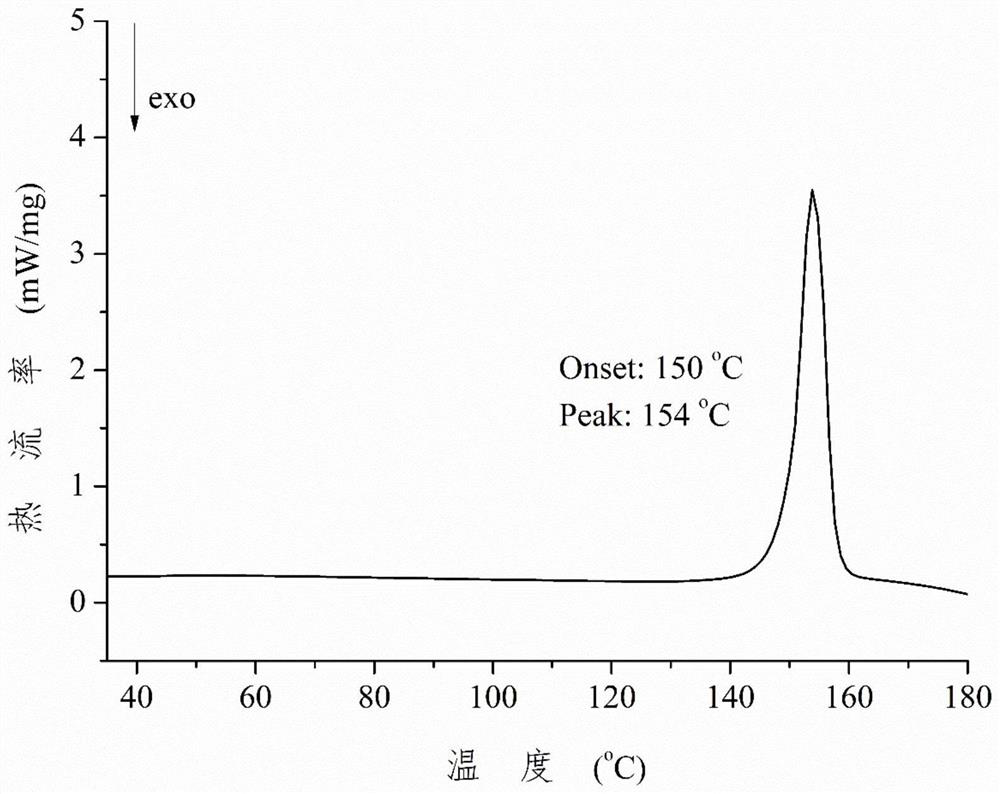
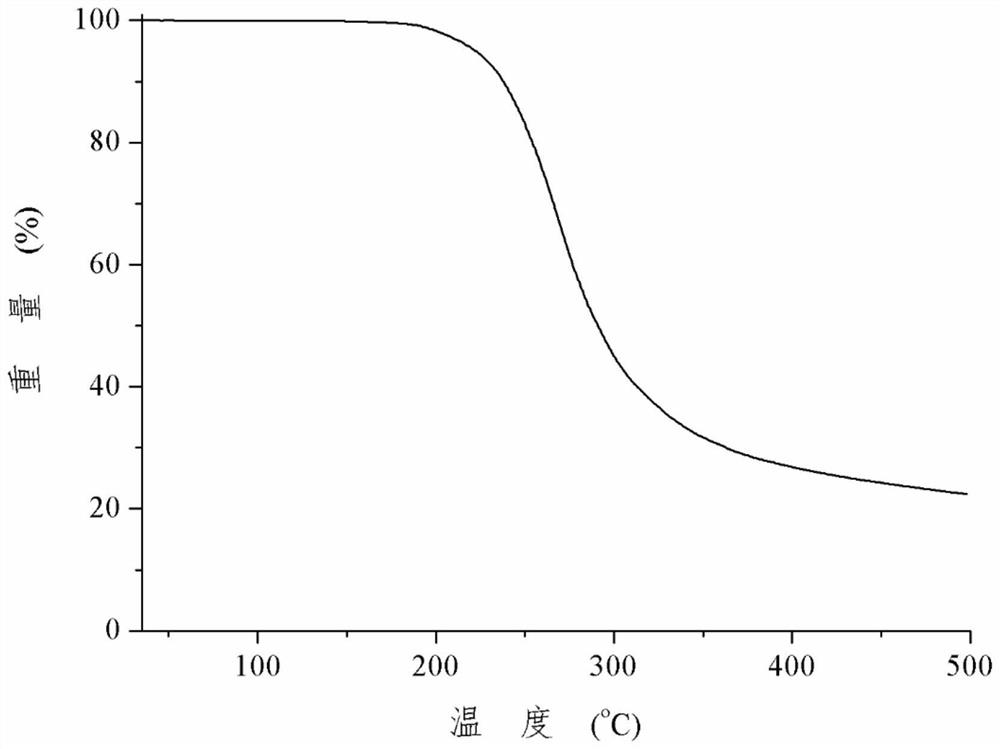
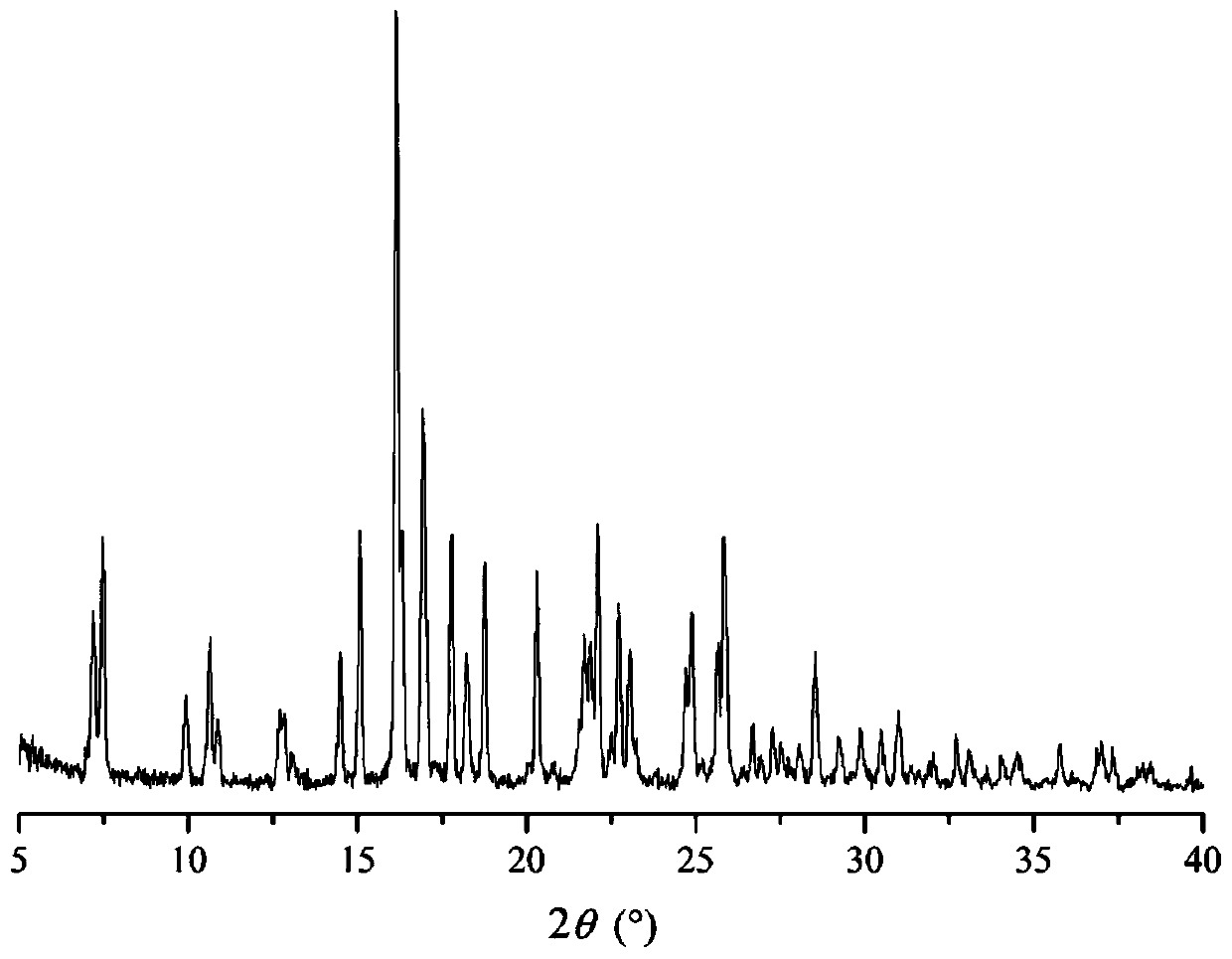
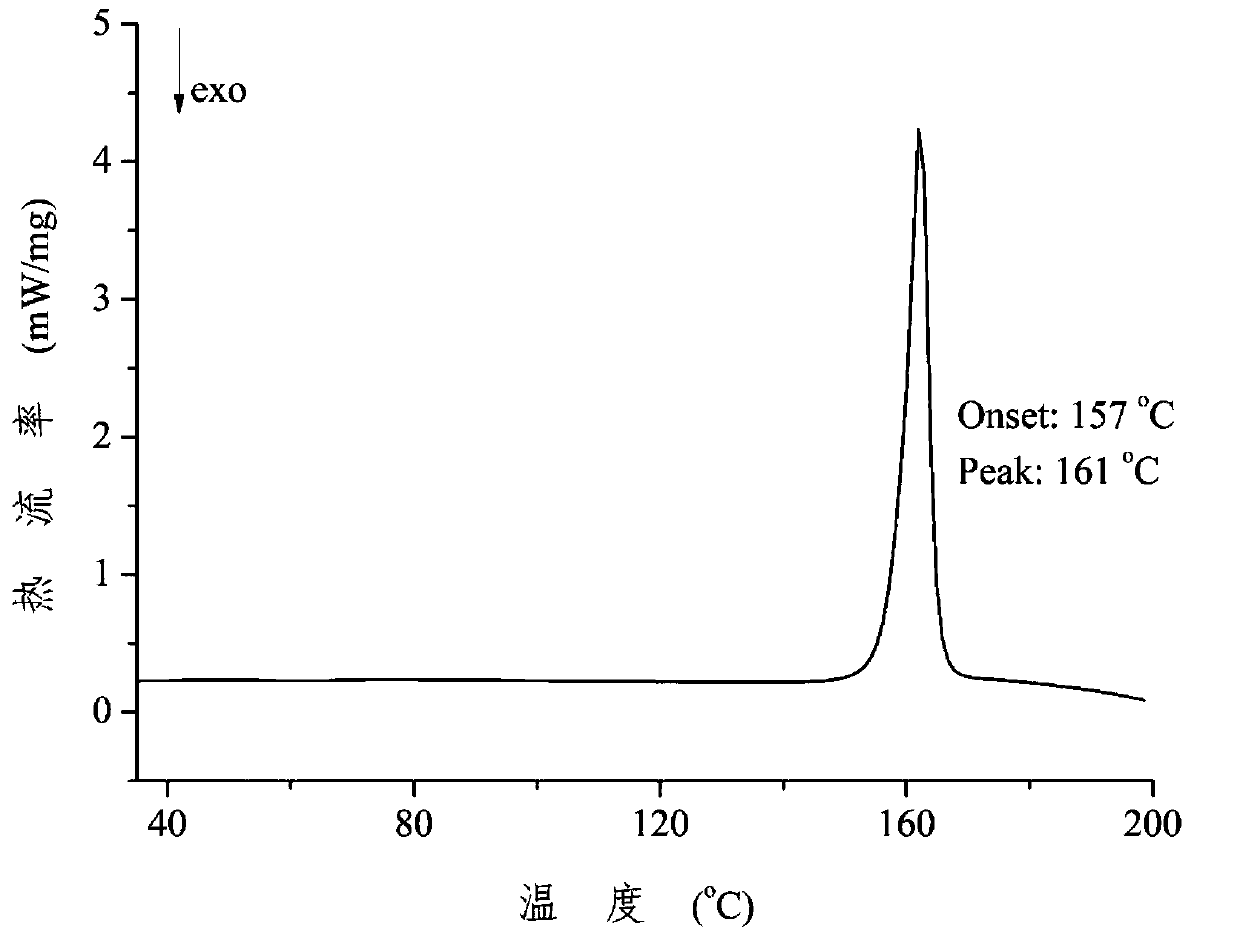
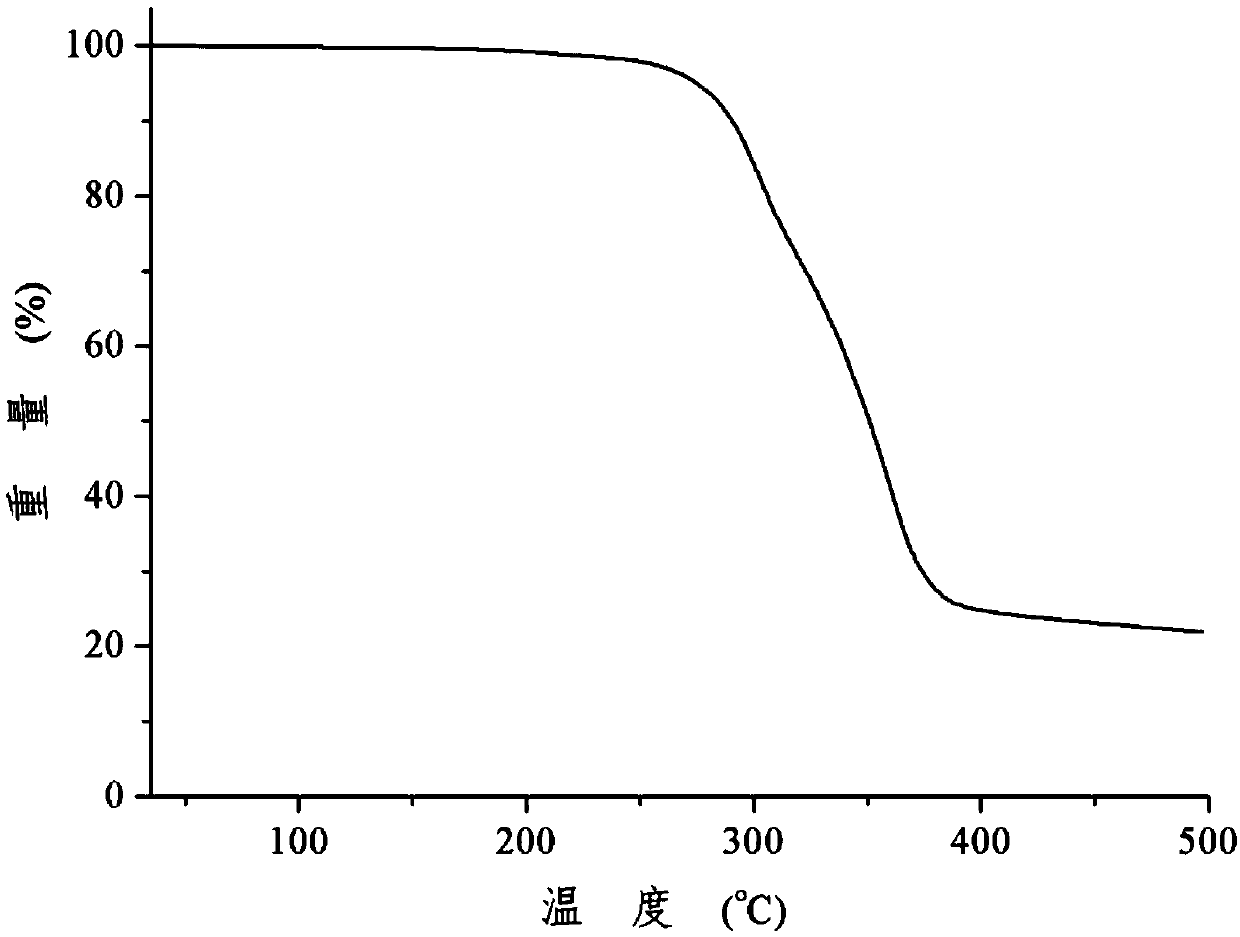
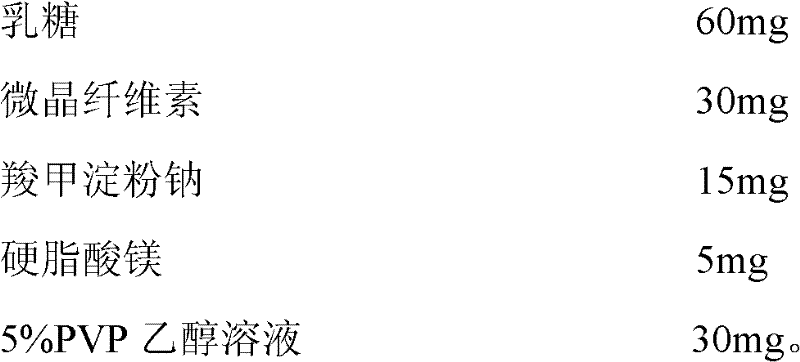
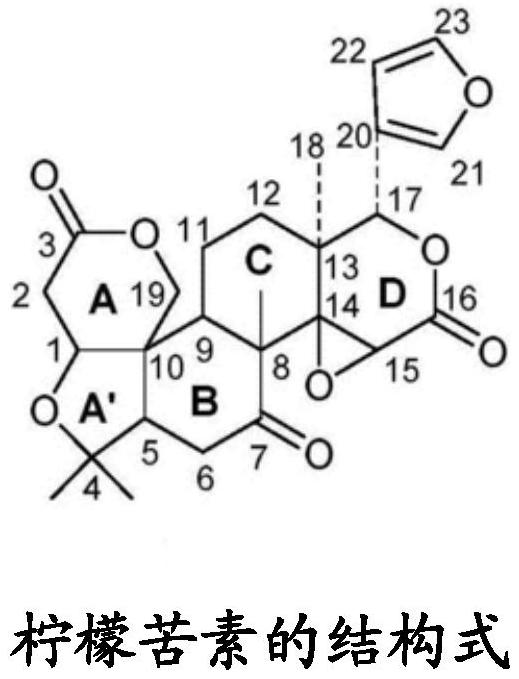
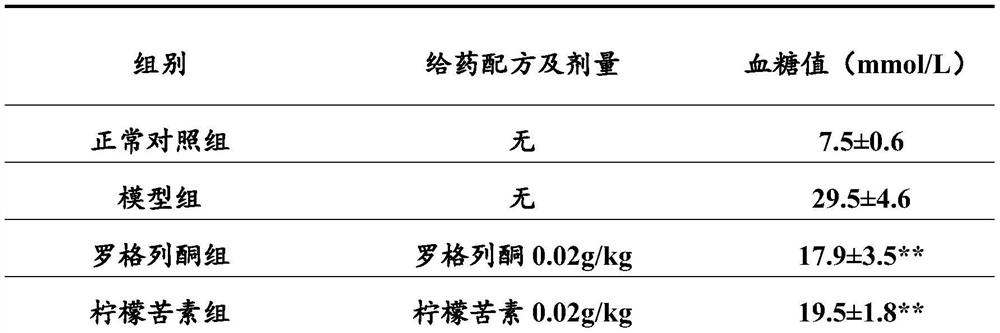
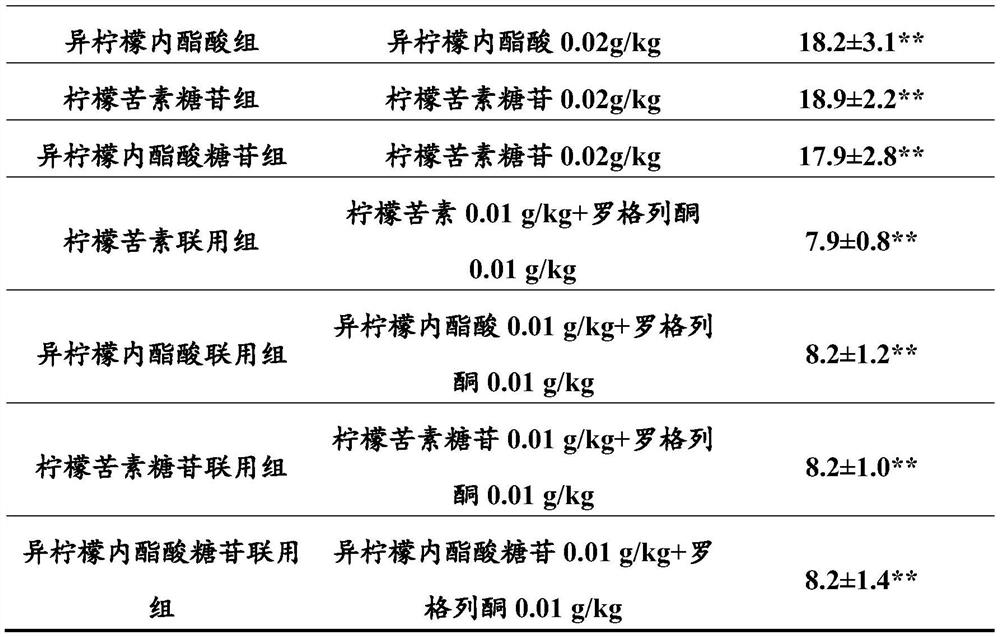
Patents
Literature
31 results about "Rosiglitazona" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of rosiglitazone and its salts
The present invention relates to rosiglitazone and its pharmaceutically acceptable salts free of at least one of the process related impurities, in particular the dehydro and the succinic acid impurities of rosiglitazone, wherein said impurities are present in an amount of about 05 mg to not more than about 15 mg, and processes for their preparation.
Owner:DR REDDYS LAB LTD +1
Hubei dwarf lilyturf tuber hetetopolysaccharide for curing type II diabete and preparation method thereof
InactiveCN101838336AProcess stabilityStable traitsOrganic active ingredientsAntimycoticsBlood sugarHigh-density lipoprotein
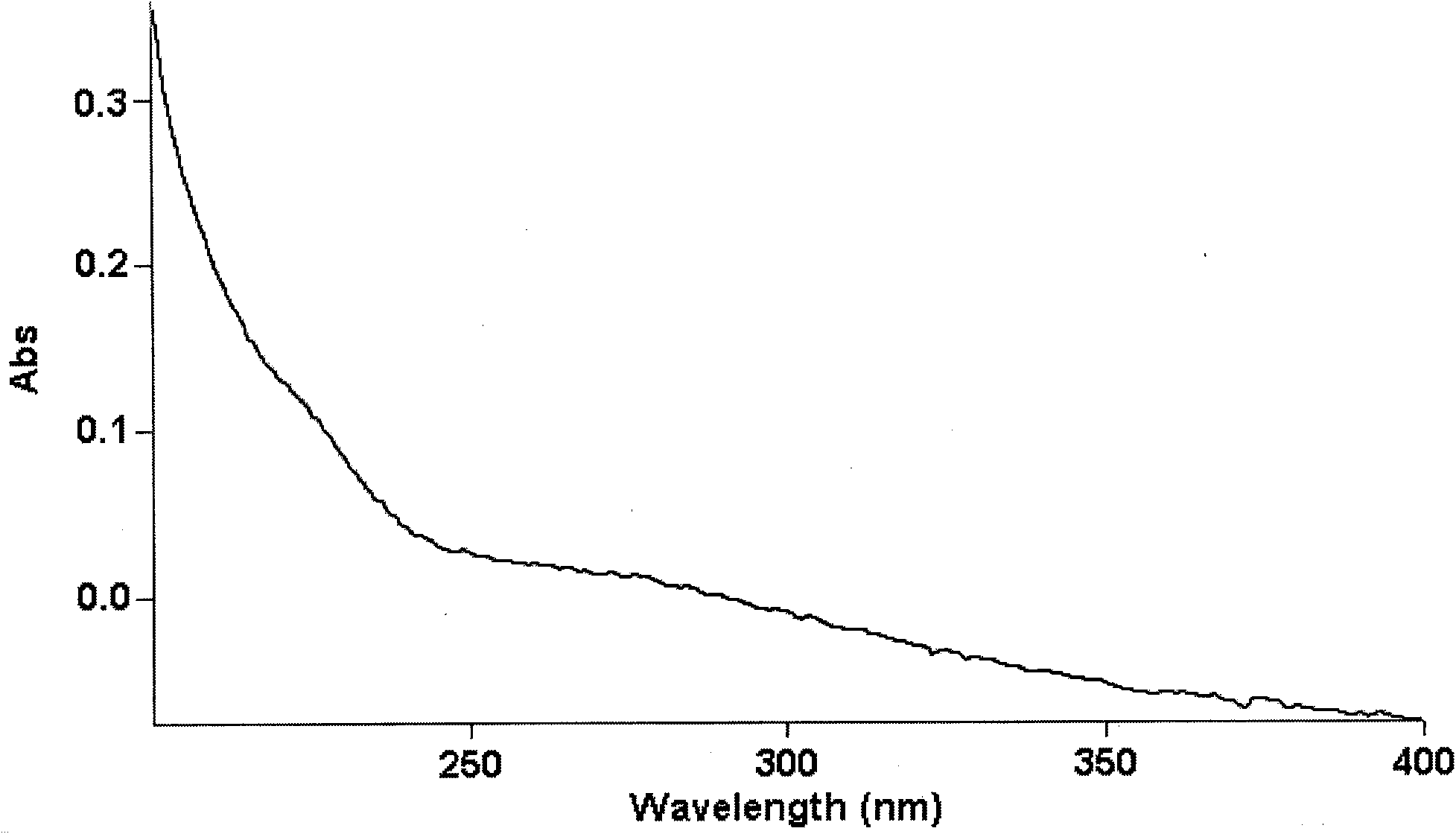
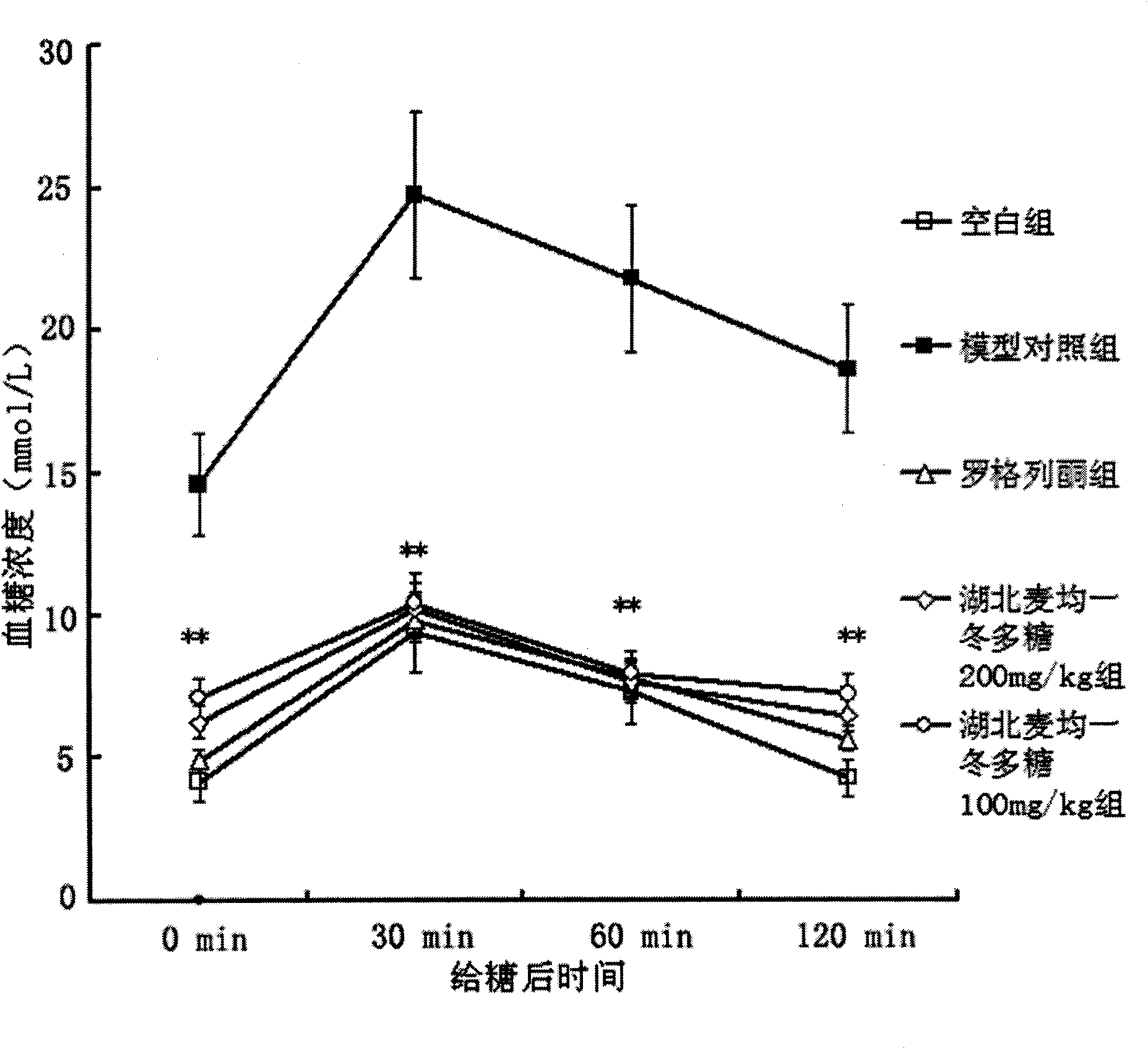
The invention provides a traditional Chinese medicine for curing type II diabete, which is Hubei dwarf lilyturf tuber hetetopolysaccharide extracted by taking Hubei dwarf lilyturf tuber as a raw material. The traditional Chinese medicine has obvious effects of reducing blood sugar of mice with the type II diabete, obvious effects of reducing insulin resistance and the effect strength of the traditional Chinese medicine has no statistical difference from that of rosiglitazone. Compared with the rosiglitazone, the hetetopolysaccharide can better reduce blood total cholesterol and triglycercide, improve ratio of high-density lipoprotein to low-density lipoprotein, and effectively prevent and curing arteriosclerosis and thrombosis.
Owner:HUAZHONG UNIV OF SCI & TECH
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
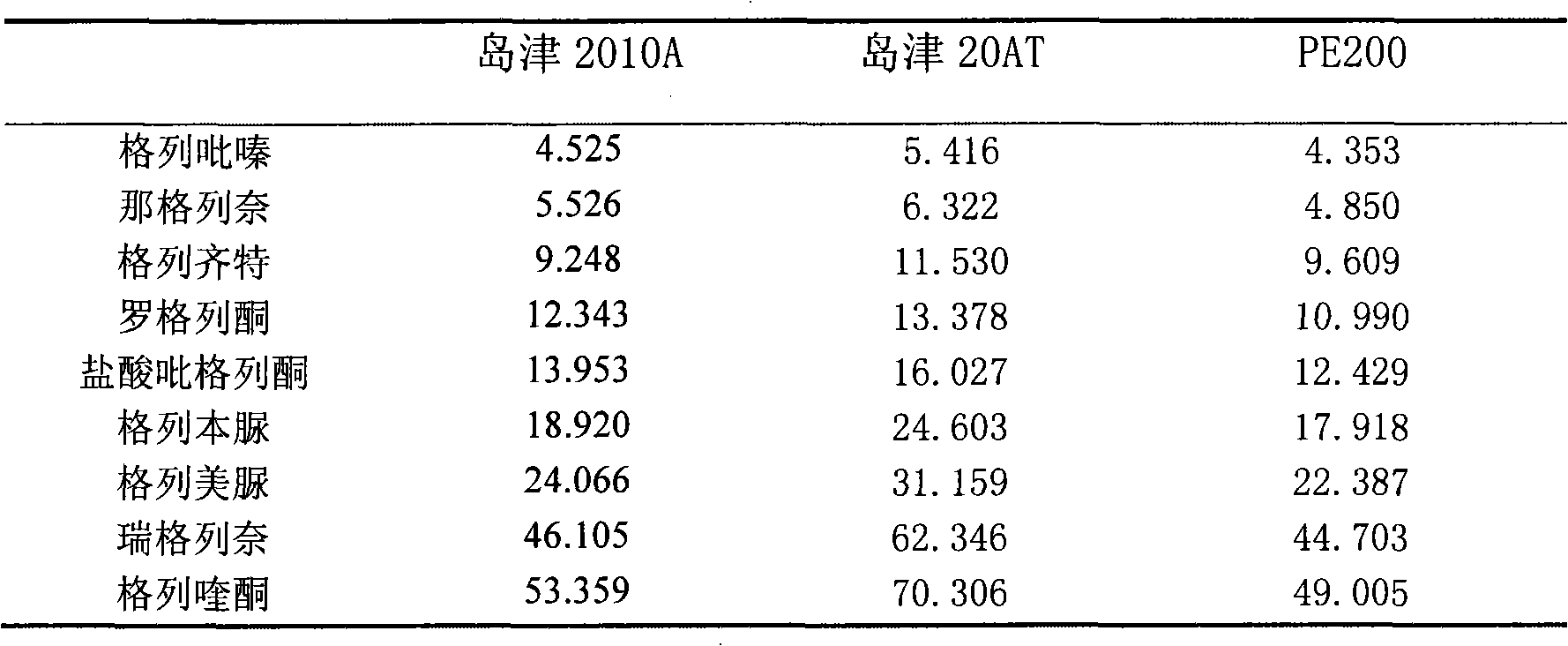
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Medicine composition for treating diabetes mellitus
ActiveCN102218062AImprove the immunityReduce dosageOrganic active ingredientsMetabolism disorderDiabetes Mellitus ComplicationsMedicine
The invention discloses a medicine composition for treating diabetes mellitus and complications thereof, and in particular relates to a medicine composition comprising arctigenin and rosiglitazone or pharmaceutically acceptable salts thereof, and application of the composition in preparation of a medicine for treating diabetes mellitus, diabetes mellitus complications and diseases relative to thediabetes mellitus.
Owner:江苏叠石桥家纺产业集团有限公司
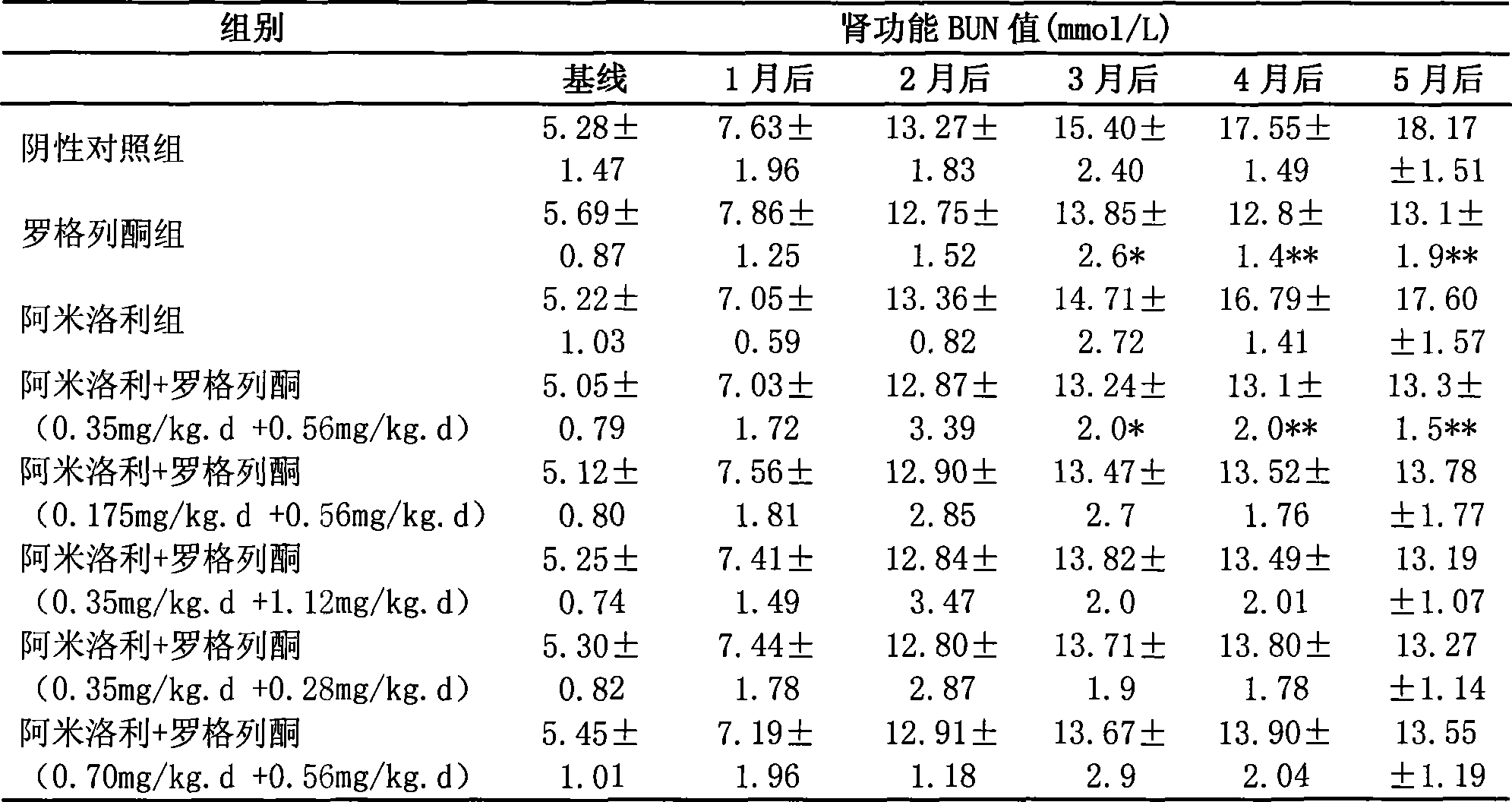
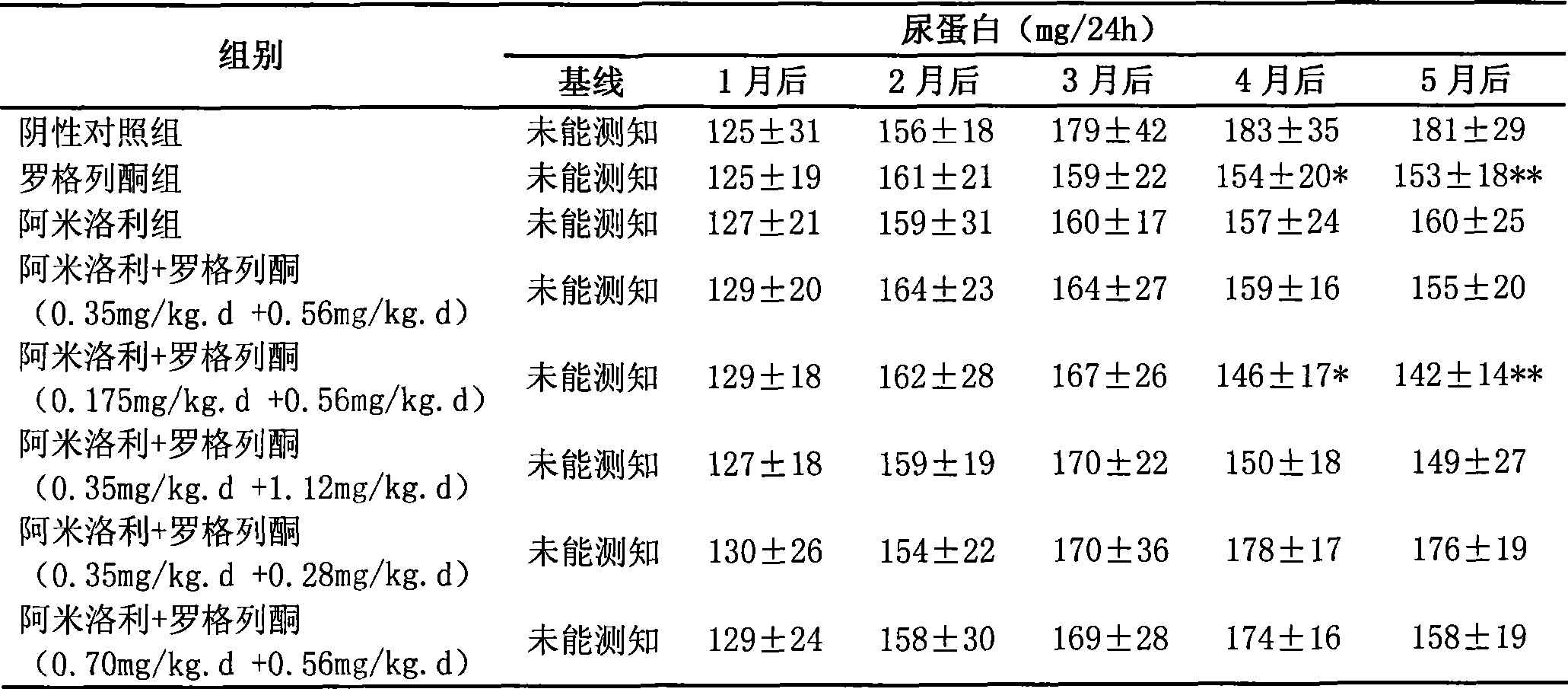
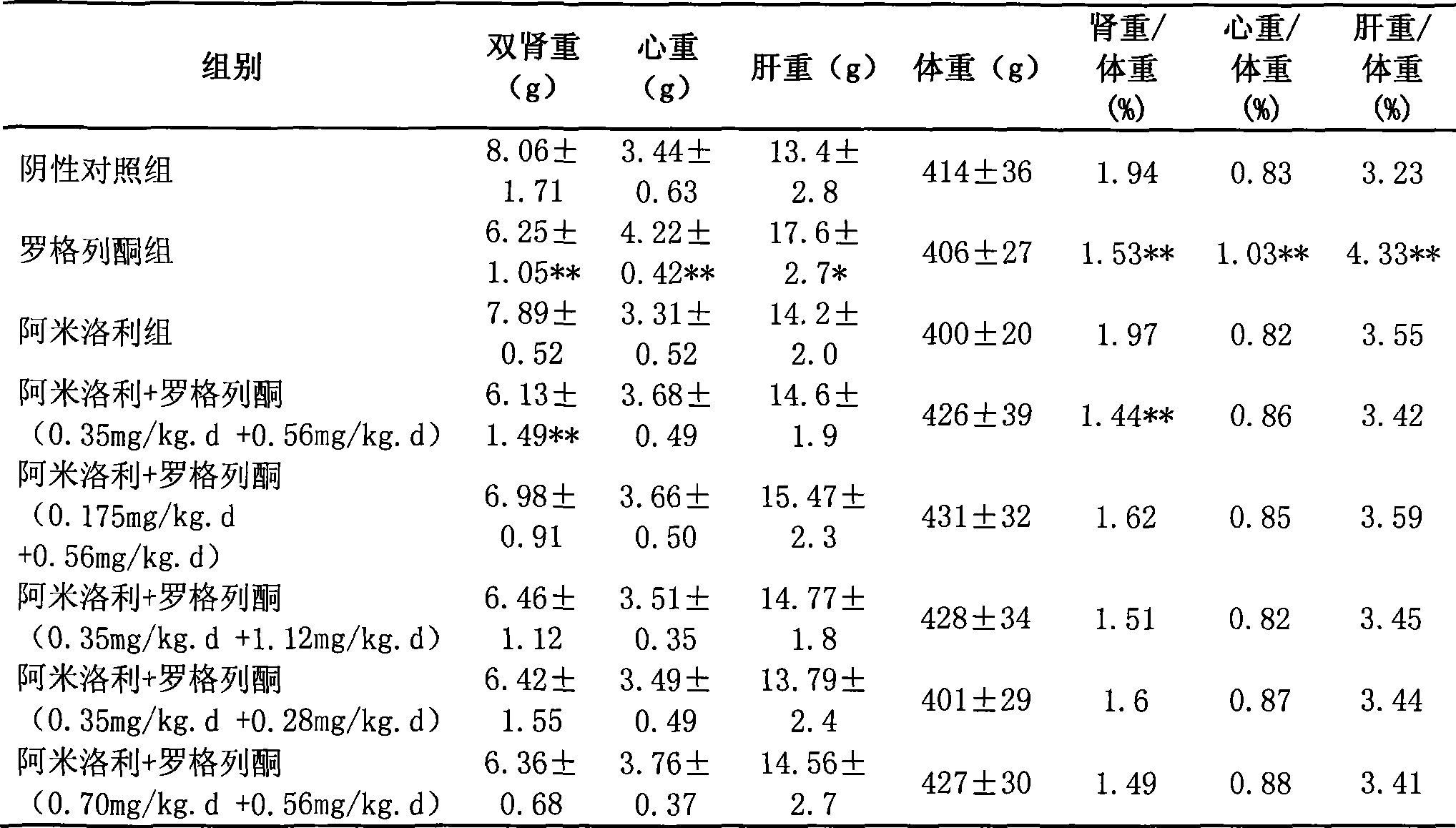
Medicine composition for treating polycystic kidney disease and use thereof
The invention relates to a pharmaceutical composition for treating polycystic kidney disease. The active ingredients of the pharmaceutical composition comprise effective doses of thiazolidinediones and diuretics. The preferential combination is as follows: the thiazolidinediones are selected from rosiglitazone and the diuretics are selected from amiloride hydrochloride. The animal experiments prove that the efficacy of the composition of the rosiglitazone and the diuretics on the treatment of the polycystic kidney disease is better than the single use of one drug. The effect of the pharmaceutical composition for treating the polycystic kidney disease is better than the single use of one drug, thereby being a drug for treating the polycystic kidney disease and being worth expecting.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Composition comprising a glucocorticoid and a thiazolidinedione for inducing compelte adipogenic differentiation of mammalian stem cells
The present invention relates to the provision of a composition comprising a glucocorticoid and a thiazolidinedione for inducing adipogenic differentiation, thereby successfully generating functional stem cells, from non-differentiated embryonic or adult stem cells originating from humans or other mammals, special preference being given to human mesenchymal stem cells. The preferred glucocorticoid is dexamethasone and the preferred thiazolidinedione is rosiglitazone. However, the invention extends to the families of both compounds.
Owner:UNIVERSIDAD DEL DESARROLLO
Non-insulin-dependent diabetes mellitus-resisting liriope spicata fructosan and preparation method and application thereof
ActiveCN101942034AProcess stabilityStable traitsOrganic active ingredientsMetabolism disorderRosiglitazoneChemistry
The invention provides liriope spicata fructosan consisting of nineteen beta-D-fructose and one alpha-D-glucose. The liriope spicata fructosan comprises a main chain consisting of fructose residues bonded at positions 1 and 2 and six repeating units, wherein each unit comprises one disubstituted fructose residue bonded at positions 1 and 2, one trisubstituted fructose residue bonded at positions 1, 2 and 6 and one monosubstituted fructose residue bonded at position 2; and hydroxyl at position 2 at one terminal of the main chain of a polysaccharide is bonded with hydroxyl at position 1 of the alpha-D-glucose to form a glycoside, and hydroxyl at position 1 at the other terminal of the main chain of the polysaccharide is bonded with hydroxyl at position 2 of one beta-D-fructose to form a glycoside. The liriope spicata fructosan has the advantages of capacity of obviously reducing the blood glucose of mice suffering from non-insulin-dependent diabetes mellitus and the insulin resistance, reducing the total cholesterol and triglyceride in blood, increasing the ratio of high-density lipoprotein to low-density lipoprotein better and more effectively preventing and treating arteriosclerosis and thrombosis, wherein the strength of the insulin resistance and that of rosiglitazone do not have any statistical difference.
Owner:HUAZHONG UNIV OF SCI & TECH
Novel pharmaceutical composition for diabetes prevention and treatment
InactiveCN103768061AMaintenance activationExcellent tolerance improvementOrganic active ingredientsMetabolism disorderSide effectTolerability
The invention aims to research a combined treatment of pioglitazone and rosiglitazone, and relate to a novel pharmaceutical composition for diabetes prevention and treatment which has an excellent function of PPAR excitation (activation) and which alleviate side effects at the same time. The PPAR excitation function can be maintained by giving the pioglitazone and rosiglitazone, preparations of acceptable slat thereof in the pharmacology, or alternatively giving the abovementioned single dosages and the acceptable slat thereof in the pharmacology. The novel pharmaceutical composition has an excellent insulin resistance function and an excellent hypoglycemic effect, and can alleviate side effects when being used as a single dosage.
Owner:王松
Application of fenticonazole nitrate to preparation of anti-diabetic drug
ActiveCN113209091AEfficient hypoglycemic effectHas low toxicity and side effectsOrganic active ingredientsMetabolism disorderSide effectDiabrezide
The invention discloses an application of fenticonazole nitrate to preparation of an anti-diabetic drug. Under the action of the concentration of 10 [mu] M, the fenticonazole nitrate has relatively strong affinity with PPAR gamma. Experiments show that compared with rosiglitazone, the fenticonazole nitrate has a relatively weak capability of inducing adipocyte differentiation, and the fenticonazole nitrate weakly activates expression of a PPAR gamma downstream adipogenic gene. In a db / db model mouse, the fenticonazole nitrate does not cause increase of the weight of the mouse and does not generate obvious side effects on all internal organs of the mouse. Besides, under the concentration of 10mg / kg, a very good hypoglycemic effect is also achieved. It is found for the first time that the fenticonazole nitrate has an efficient hypoglycemic effect, has low toxic and side effects, and can be developed as an anti-diabetic drug.
Owner:GUANGZHOU MEDICAL UNIV
Subcutaneous implant rod for long-acting blood sugar reduction, and preparation method of subcutaneous implant rod
PendingCN113440473AShort resolution timeEasy to prepareMetabolism disorderSulfonylurea active ingredientsPolythylene glycolPyrrolidinones
The invention discloses a subcutaneous implant rod for long-acting blood sugar reduction, and a preparation method of the subcutaneous implant rod. A diblock copolymer is adopted as a carrier, and the formula comprises the following substances in parts by weight: 10-20 parts of polycaprolactone, 5-10 parts of polyethylene glycol, 1-5 parts of polyvinylpyrrolidone and 5-20 parts of bulk drugs, wherein the bulk drug is prepared by combining two drugs, namely glibenclamide and rosiglitazone, and the mass ratio of the glibenclamide to the rosiglitazone in the bulk drug is (1-20): 1. After the subcutaneous implant rod disclosed by the invention is implanted subcutaneously, direct long-term sustained-release administration is carried out for realizing long-term treatment and stabilization of blood sugar, the diblock copolymer is adopted as the carrier, the problem that an existing implant rod carrier is short in acting time is solved, the preparation method of the subcutaneous implant rod disclosed by the invention is simple, materials can be easily obtained, and the production process is simple and can be easily mastered. The subcutaneous implant rod prepared by the invention is implanted under the skin of the human body, and a comfort level is good after the subcutaneous implant rod prepared by the invention is implanted under the skin of the human body since a biological material has complete degradability, good biocompatibility and no toxicity.
Owner:吕汇川
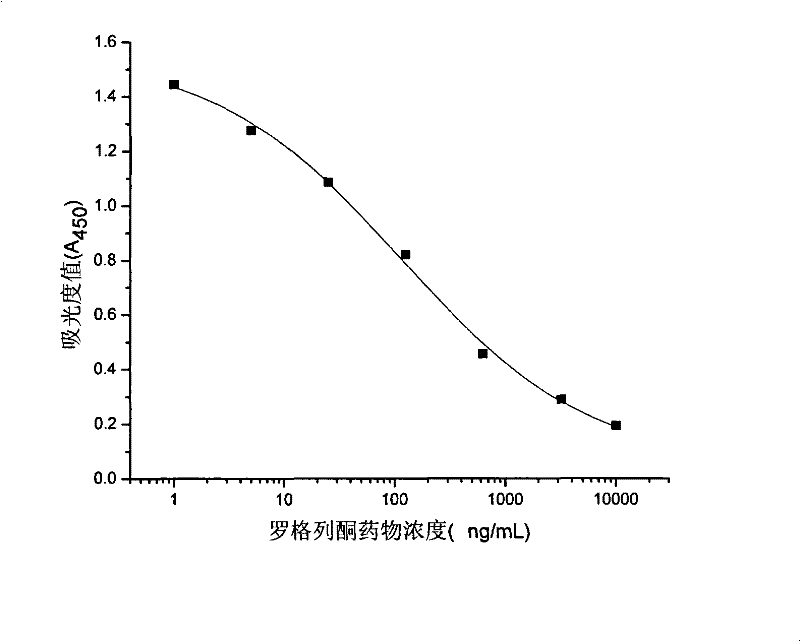
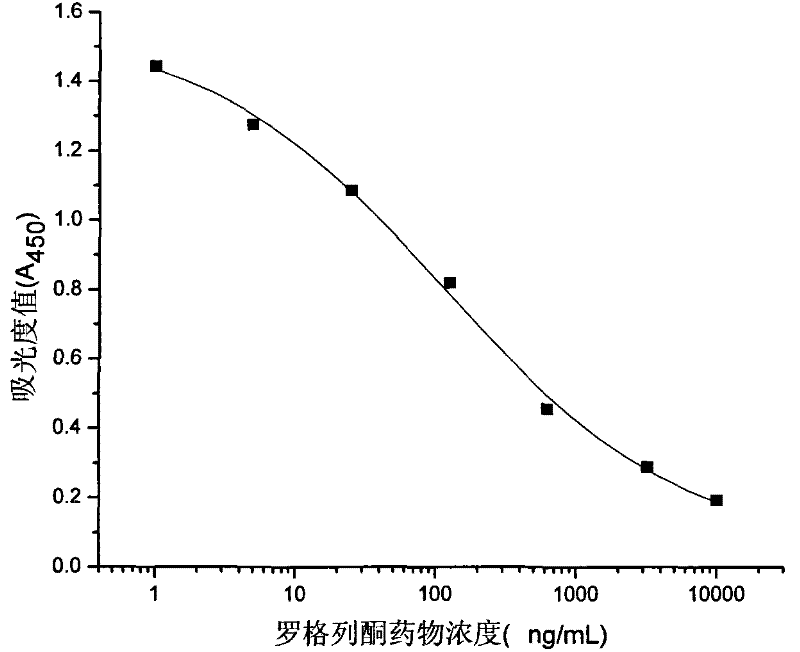
Rogridone hapten, artificial antigen and antibody as well as preparation method and application thereof
ActiveCN101538266BReduce testing costsHigh sensitivityOvalbuminSerum albuminBenzyl benzoatFood safety
The invention discloses a rogridone hapten as well as a corresponding artificial antigen and an antibody thereof. The invention also discloses a preparation method and an application for the rogridone hapten as well as the corresponding artificial antigen and the antibody. The method comprises the following steps: adopting rogridone as a raw material which reacts with halogenated benzyl benzoate to generate the carboxyl-contained rogridone hapten; then, coupling the rogridone hapten and protein by an activated fat method or a mixed acid anhydride method to prepare the rogridone artificial antigen; and using the rogridone artificial antigen to immunize animals so as to generate the high-specificity rogridone antibody. An immunodetection method for the rogridone antibody and the rogridone artificial antigen can be applied to the on-site rapid detection of the rogridone and has an important realistic meaning to realize the rapid detection of the health food safety.
Owner:华农(潮州)食品研究院有限公司
Solid pharmaceutical composition containing metformin and vildagliptin
InactiveCN112691095AInhibition of complicationsMetabolism disorderPill deliveryUse medicationCaplet Dosage Form
The invention relates to a solid pharmaceutical composition containing metformin and vildagliptin. The solid pharmaceutical composition containing metformin and vildagliptin comprises the following components in parts by mass: 1-3 parts of the metformin, 15-60 parts of the vildagliptin, 1-3 parts of rosiglitazone and 0.8-2 parts of vitamin B. The solid pharmaceutical composition containing metformin and vildagliptin is a single tablet or a capsule. According to the solid pharmaceutical composition containing metformin and vildagliptin, through scientific combination of three different hypoglycemic drugs, the purposes of mechanism complementation, mutual cooperation, curative effect improvement and reasonable medication are achieved.
Owner:江苏宇锐医药科技有限公司 +1
Combination product containing limonoid compound and thiazolidinedione compound
The present invention relates to a combination product comprising a limonoid compound (or a pharmaceutically acceptable derivative, ester, stereoisomer, salt or prodrug thereof), and a thiazolidinedione compound (e.g., rosiglitazone, pioglitazone and the like). The present invention further relates to a use of the combination product for prevention and / or treatment of a disease associated with diabetes and the like.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Combinations with thiazolidinediones for use in the prevention or treatment of abnormal bone growth
PendingUS20220288044A1Improve the blocking effectPromote differentiationOrganic active ingredientsSkeletal disorderThiazoleNon steroid anti inflammatory drug
The present invention refers to the use of thiazolidinediones (preferably rosiglitazone and / or pioglitazone) in the prevention or treatment abnormal bone growth selected from the list comprising: heterotopic ossification, osteophytes and / or syndesmophytes. According to the present invention, thiazolidinediones can be combined with corticoids and / or anti-inflammatory drugs (preferably non-steroidal anti-inflammatory drugs).
Owner:FUNDACION INST DE INVESTIGACION SANITARIA DE SANTIAGO DE COMPOSTELA +1
A kind of medicinal composition with fat-reducing effect and application thereof
ActiveCN112220924BEffectively causing deathEffectively induce lipolysisOrganic active ingredientsEnergy modified materialsTemoporfinChlorin e6
The invention belongs to the technical field of weight-loss drugs, and provides a pharmaceutical composition with a fat-reducing effect, including a photosensitizer and rosiglitazone; wherein the photosensitizer includes photoporphyrin, verteporfin, temoporfin, One of chlorin e6, zinc phthalocyanine, and 5-aminolevulinic acid or a mixture of them; the mass ratio of the photosensitizer to rosiglitazone is 3:1-10:1. During administration, the pharmaceutical composition powder of the present invention is diluted and dissolved with physiological saline, and then administered through intravenous injection. The present invention combines the photodynamic cell killing of the photosensitizer with the browning induction of rosiglitazone to realize the optimal weight loss strategy combining "fast-slow" and "local-whole". The two drugs are a good complementary pair, and the experimental results show that they have quite good synergistic effects; in addition, rosiglitazone also has a significant inhibitory effect on the adverse reactions that may be caused by photosensitizer treatment.
Owner:XIAMEN UNIV
A kind of rosiglitazone nano-preparation and its preparation method and application
InactiveCN109481419BImprove permeabilityGood dispersionOrganic active ingredientsAntipyreticPolythylene glycolPhospholipid
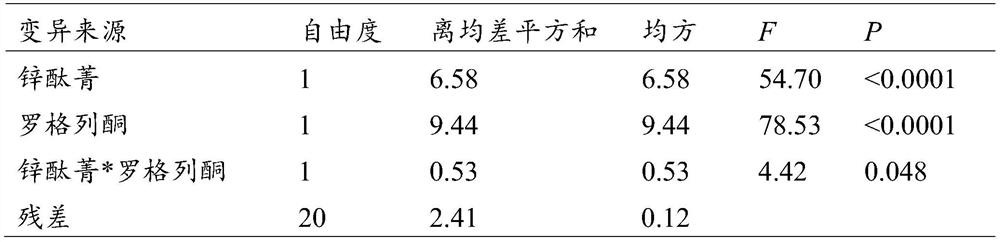
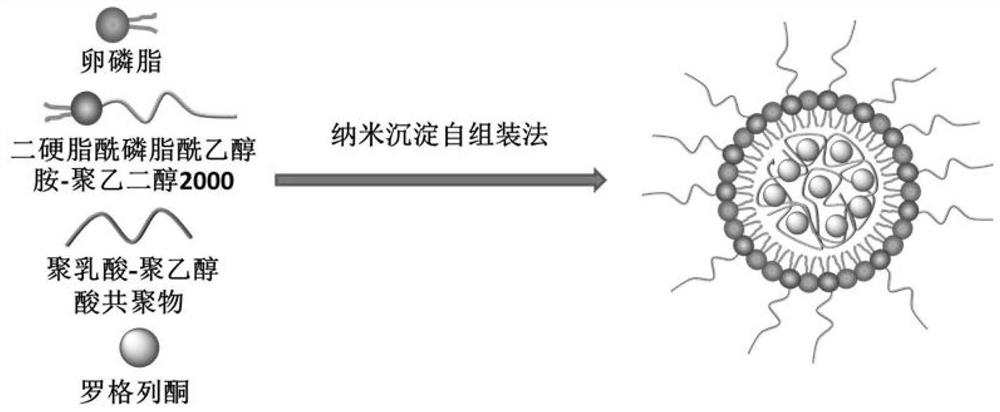
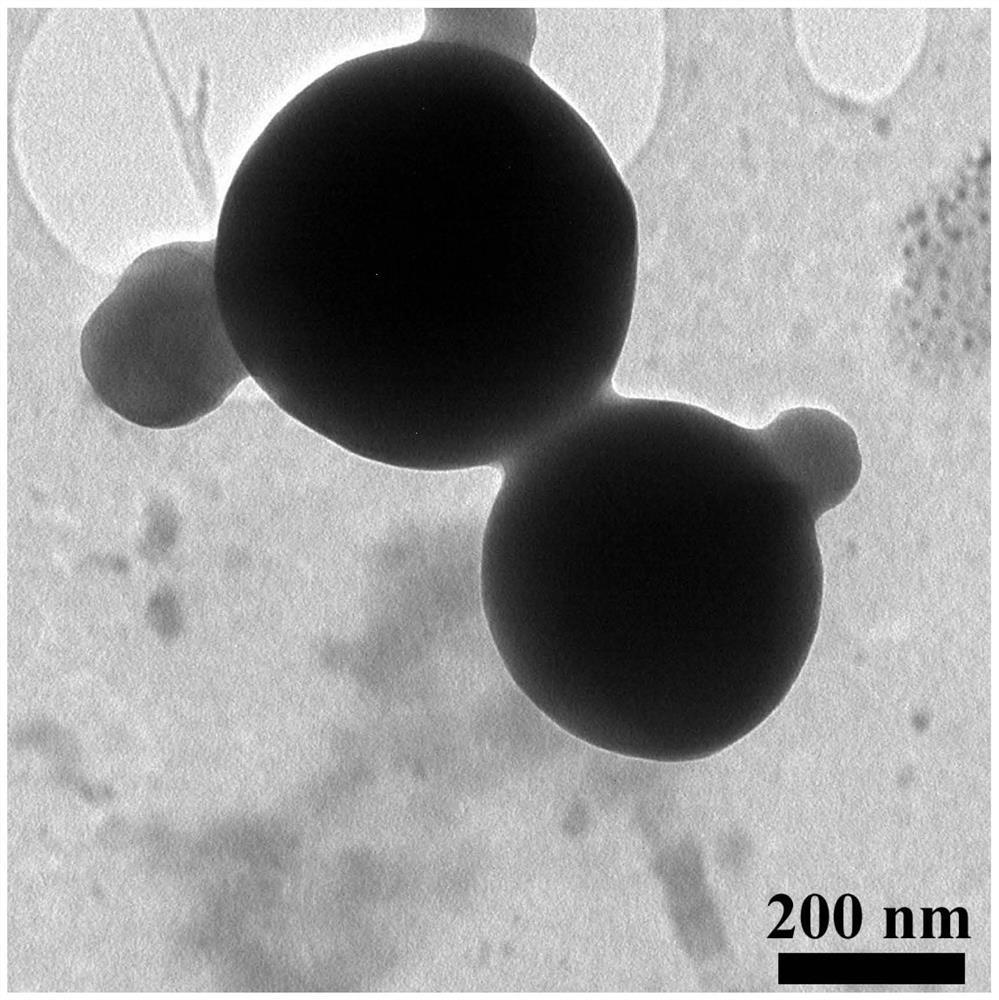
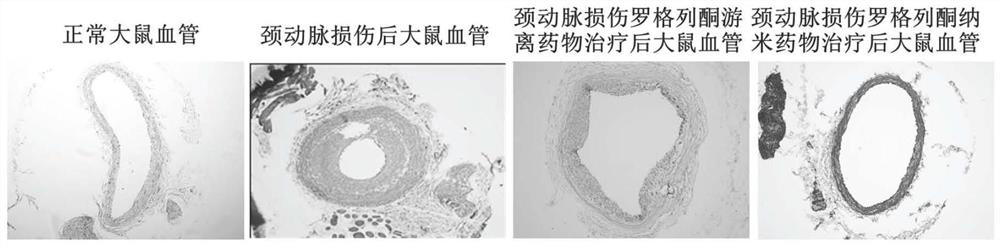
The invention discloses an application of a rosiglitazone nano preparation in treating vascular restenosis. A rosiglitazone composition contains rosiglitazone, a polylactic acid-polyglycolic acid copolymer, lecithin and distearyl phosphatidyl ethanolamine-polyethylene glycol 2000. A rosiglitazone nano preparation is nano particles formed by rosiglitazone, polylactic acid-polyglycolic acid copolymer, lecithin and distearyl phosphatidyl ethanolamine-polyethylene glycol 2000, the structure is of a spherical structure, and the particle size is between 250 nm and 300 nm. The invention also discloses a preparation method of the rosiglitazone nano preparation, and the rosiglitazone nano preparation can effectively treat vascular restenosis after implanting of an intravascular stent, and can reduce the toxicity of rosiglitazone and improve the biological safety.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Impurity removal method of rosiglitazone hydrochloride
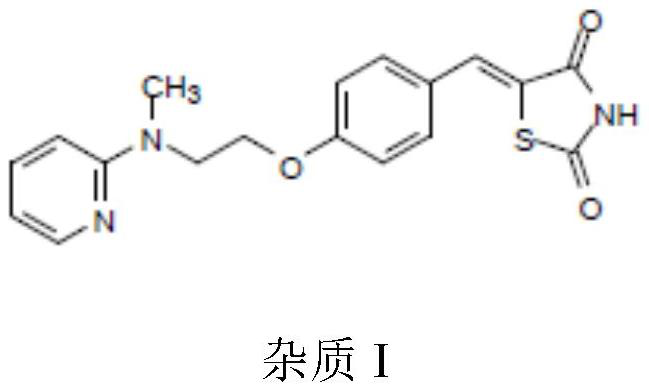
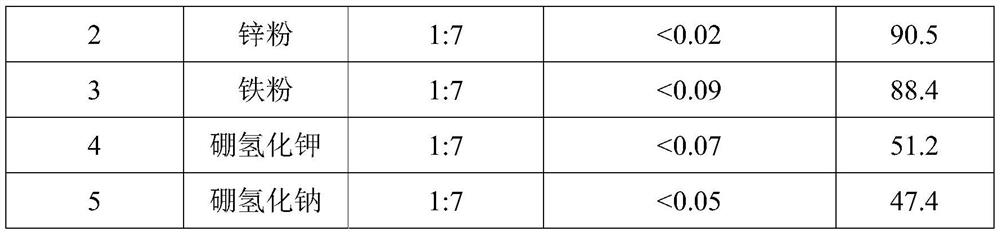
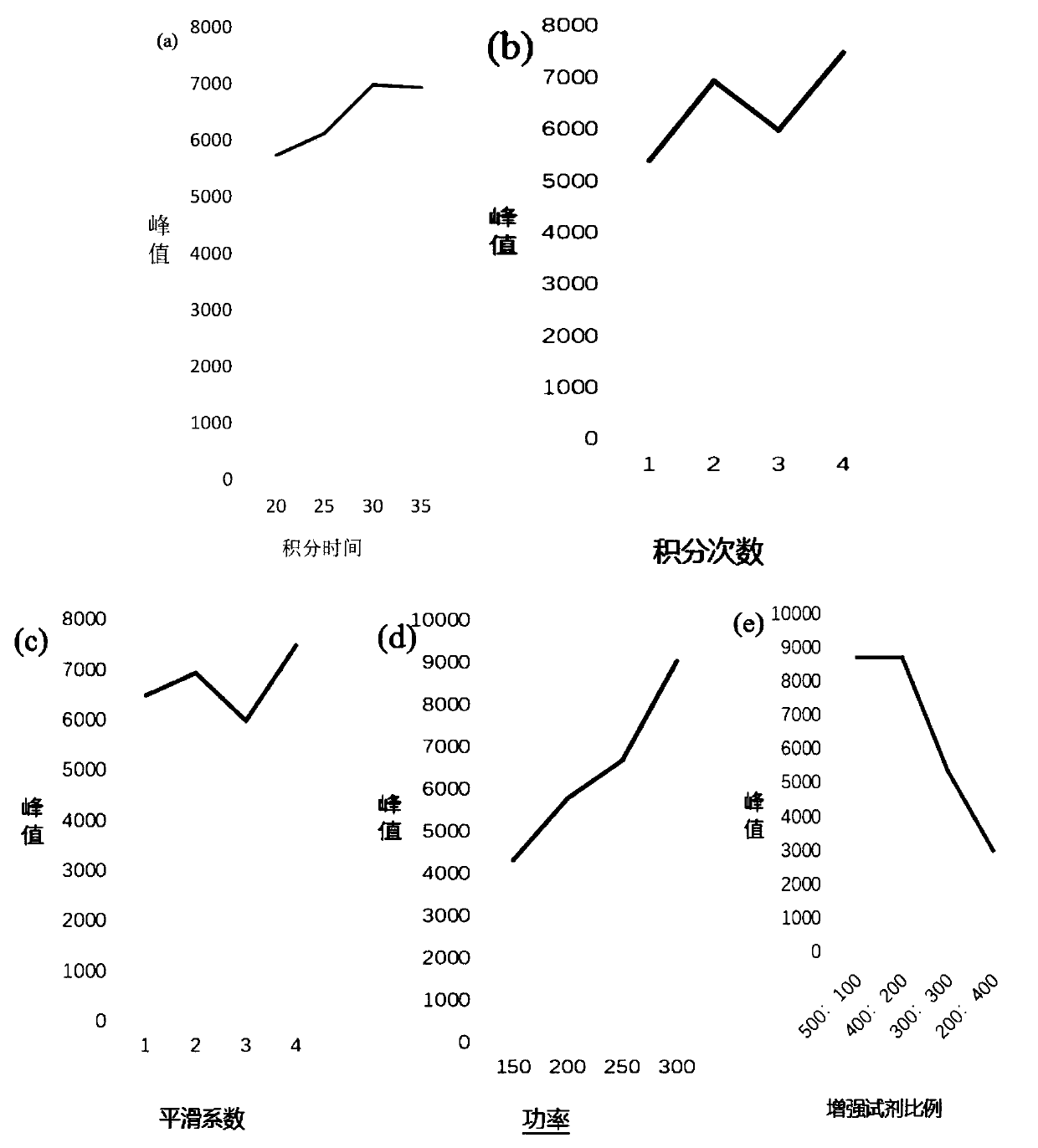
The invention belongs to the technical field of medicine synthesis, and particularly relates to an impurity removal method of rosiglitazone hydrochloride. According to the method, a reducing agent is added into a rosiglitazone hydrochloride crude product and can react with residual hydrogen ions in the crude product, hydrogen atoms with high reducibility are generated and react with an impurity I, the impurity I can be consumed to a great extent and even completely consumed, and the effect of remarkably reducing the impurity I can be achieved without repeated impurity removal; meanwhile, relatively high yield can be kept; and the impurity removal method is simple to operate, mild in condition, low in cost and suitable for large-scale industrial production.
Owner:RUYUAN HEC PHARM
Qualitative detection method for pioglitazone and rosiglitazone in food and medicine
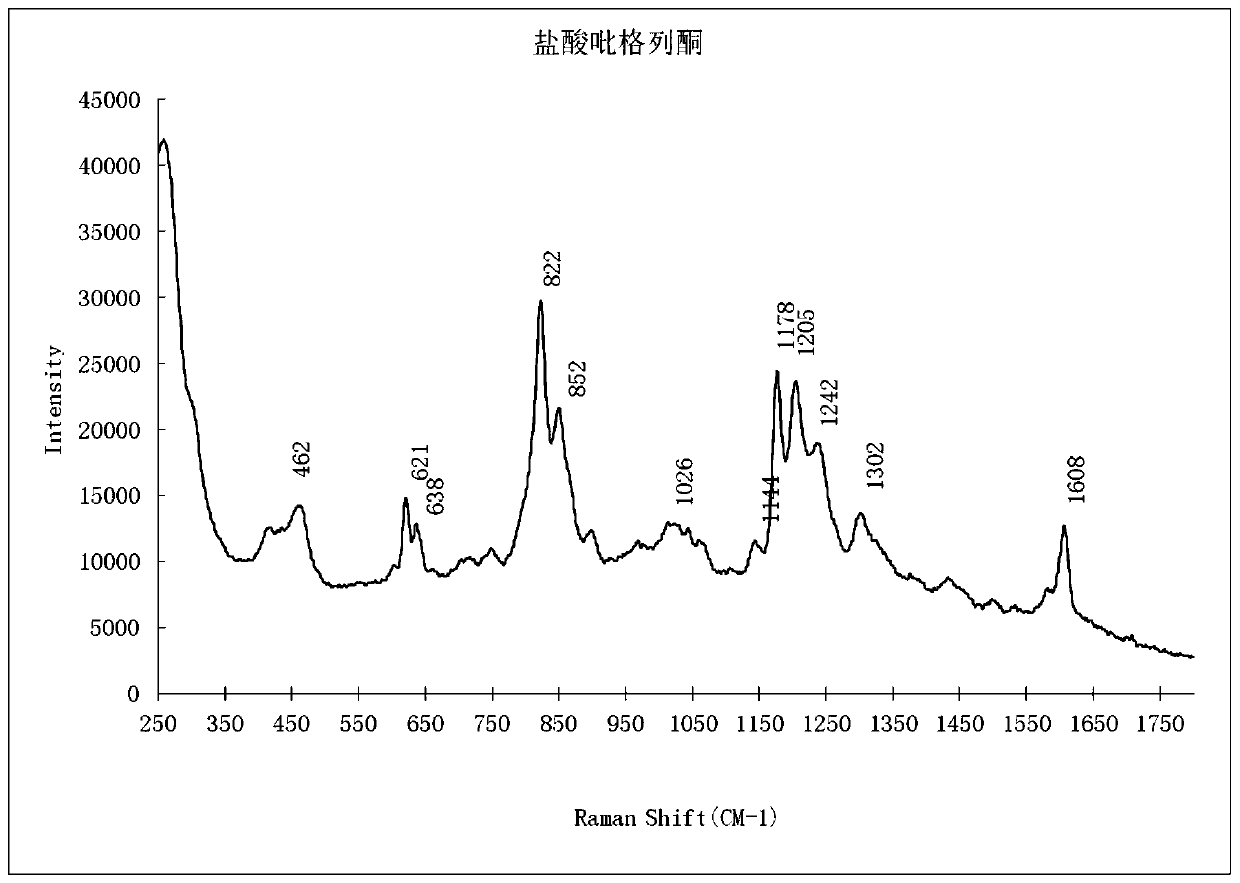
PendingCN111208112AEnhanced Raman effectReduce distractionsPreparing sample for investigationRaman scatteringBiotechnologySurface-enhanced Raman spectroscopy
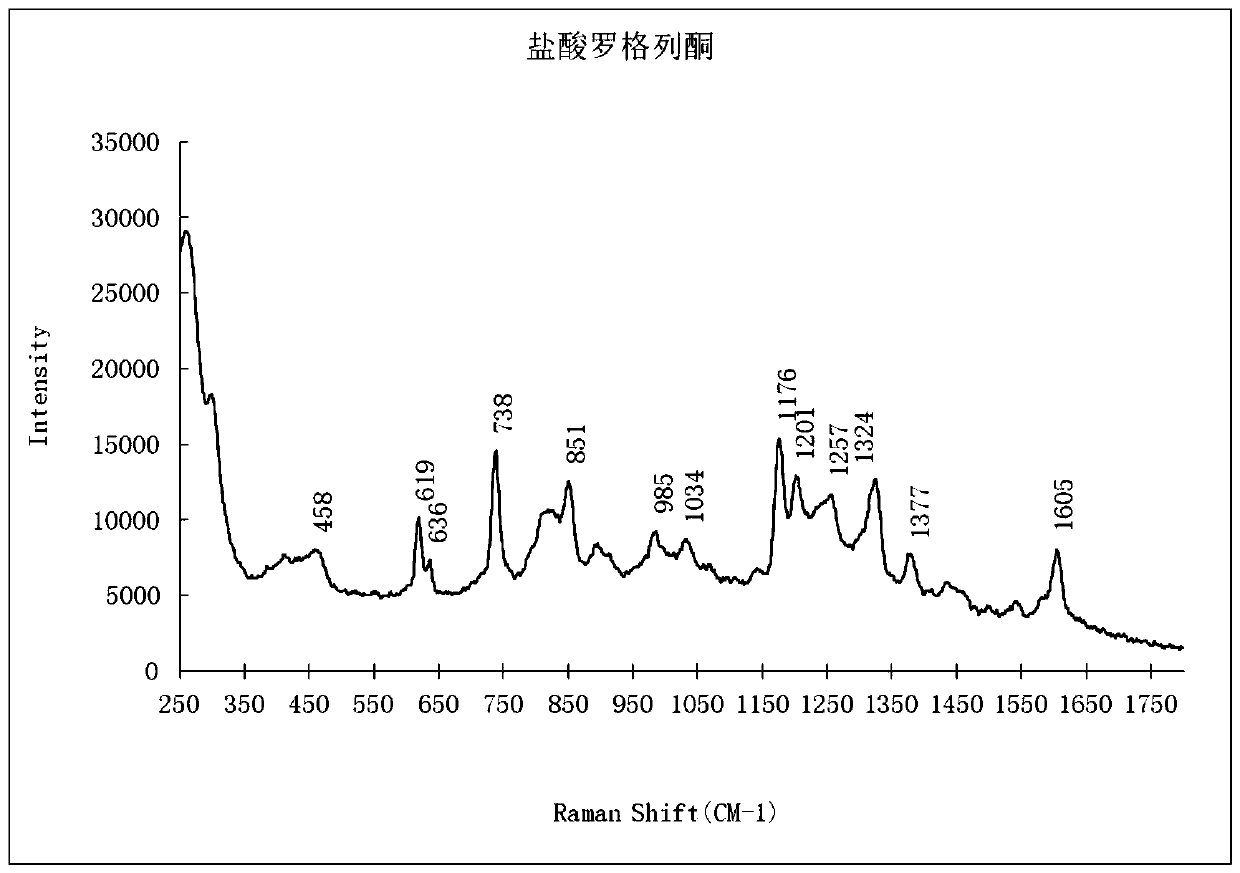
The invention provides a qualitative detection method for pioglitazone and rosiglitazone in foods and medicines and belongs to the field of substance detection. The method is characterized in that twotypes of gold nanoparticles are proportioned to serve as nanoparticle enhancing reagent, water-saturated trichloromethane serves as extracting agent for extraction, acid is not needed for extraction,the method is safer, interference is reduced, the Raman effect of a target object is enhanced, the characteristics are more obvious and the strength is higher under the same concentration, and then accuracy of the detection result can be improved. Data of the embodiment show that the method is suitable for detecting the illegally added pioglitazone and the rosiglitazone in the hypoglycemic health-care food by surface enhanced Raman spectroscopy, and detection limits of the method are both 15 mu g / mL.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
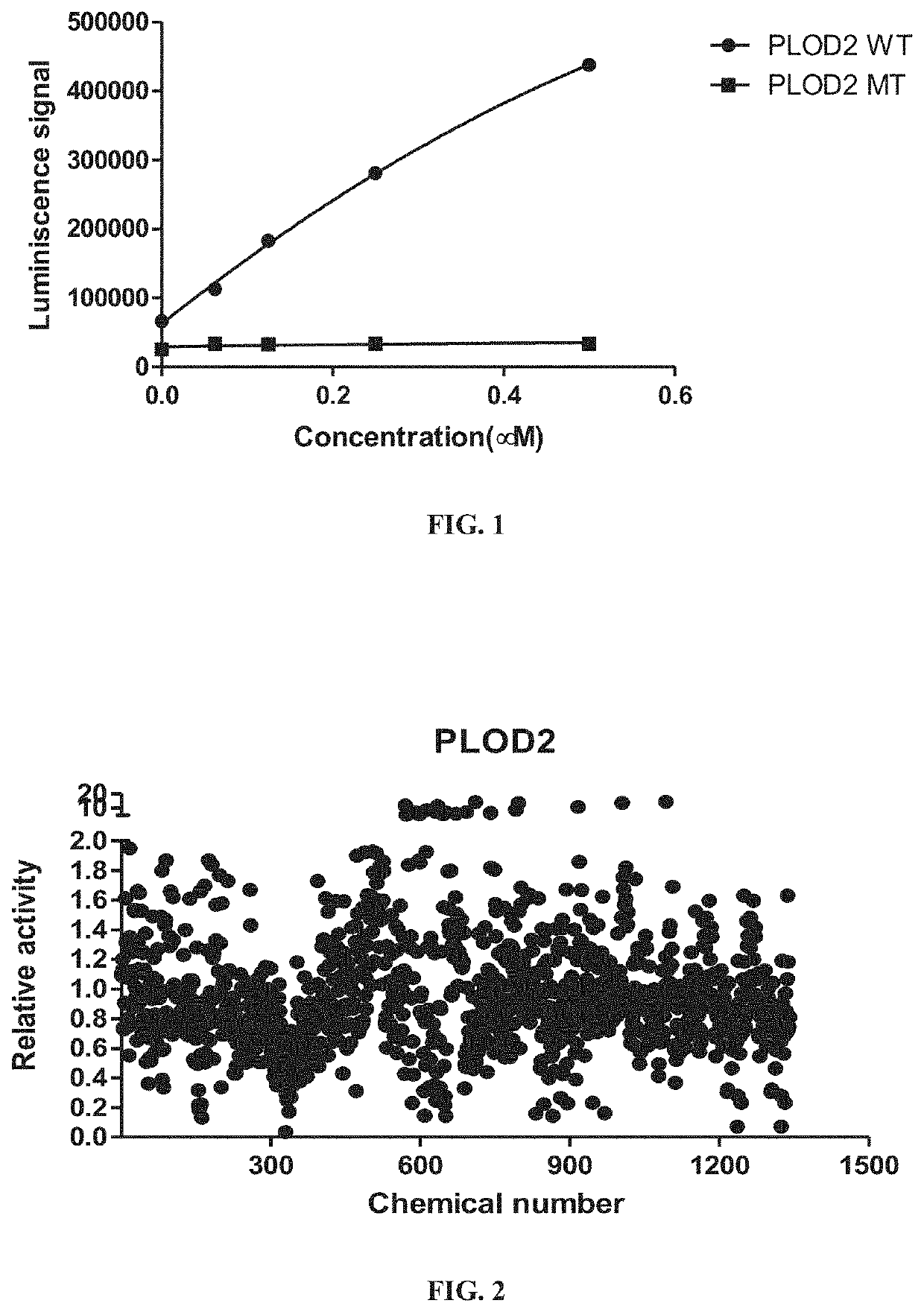
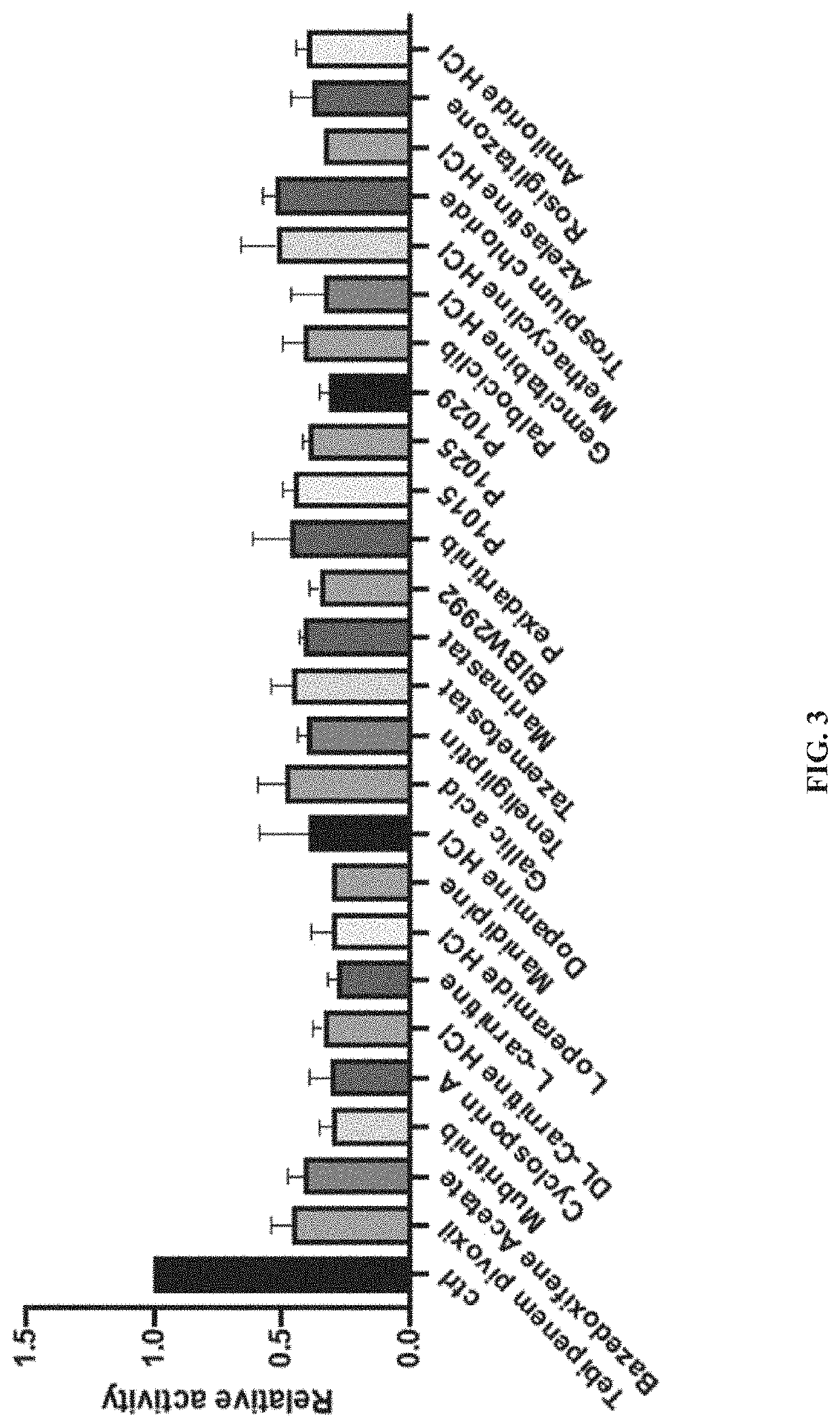
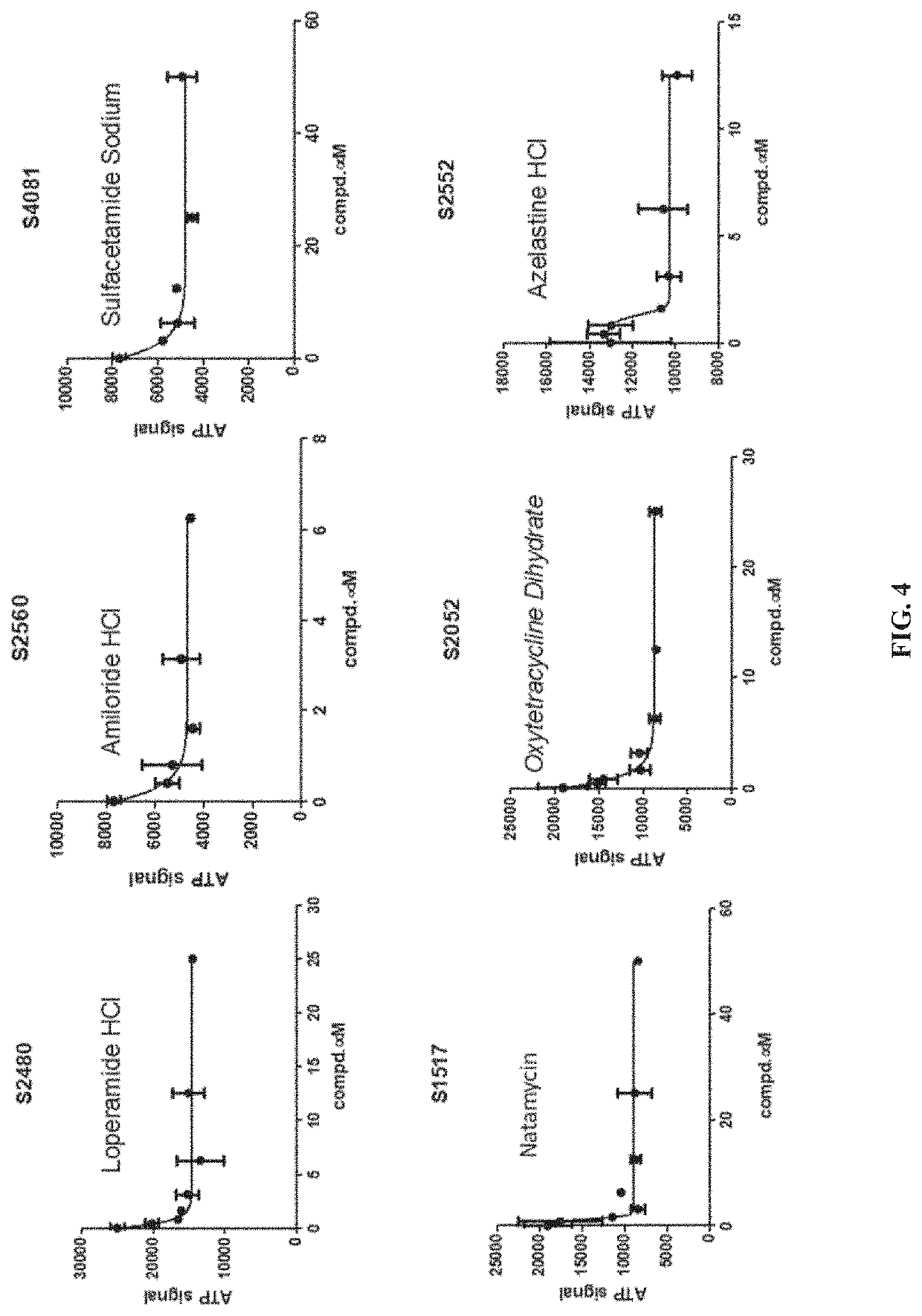
Methods of inhibiting procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2
PendingUS20220031668A1Inhibit cell migrationTetracycline active ingredientsCyclic peptide ingredientsAzelastineTEBIPENEM PIVOXIL
A method of inhibiting expression or activity of procollagen-lysine, 2-oxoglutarate 5-dioxygenases 2 (PLOD2) in a cell involves contacting the cell with or introducing into the cell an effective amount of a compound selected from the group consisting of: amiloride, azelastine, bazedoxifene acetate, BIBW2992, DL-carnitine, L-carnitine, cyclosporin A, dopamine, gallic acid, gemcitabine, loperamide, manidipine, marimastat, methacycline, mubritinib, P1015, P1025, P1029, palbociclib, pexidartinib, rosiglitazone, tazemetostat, tebipenem pivoxil, teneligliptin, trospium chloride, and pharmaceutically-acceptable salts thereof.
Owner:UNIV OF KENTUCKY RES FOUND
A kind of rosiglitazone saccharin salt and preparation method thereof
ActiveCN109053718BGood dissolution propertiesGreat tasteOrganic active ingredientsMetabolism disorderPharmaceutical industryPhysical chemistry
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample tobe tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
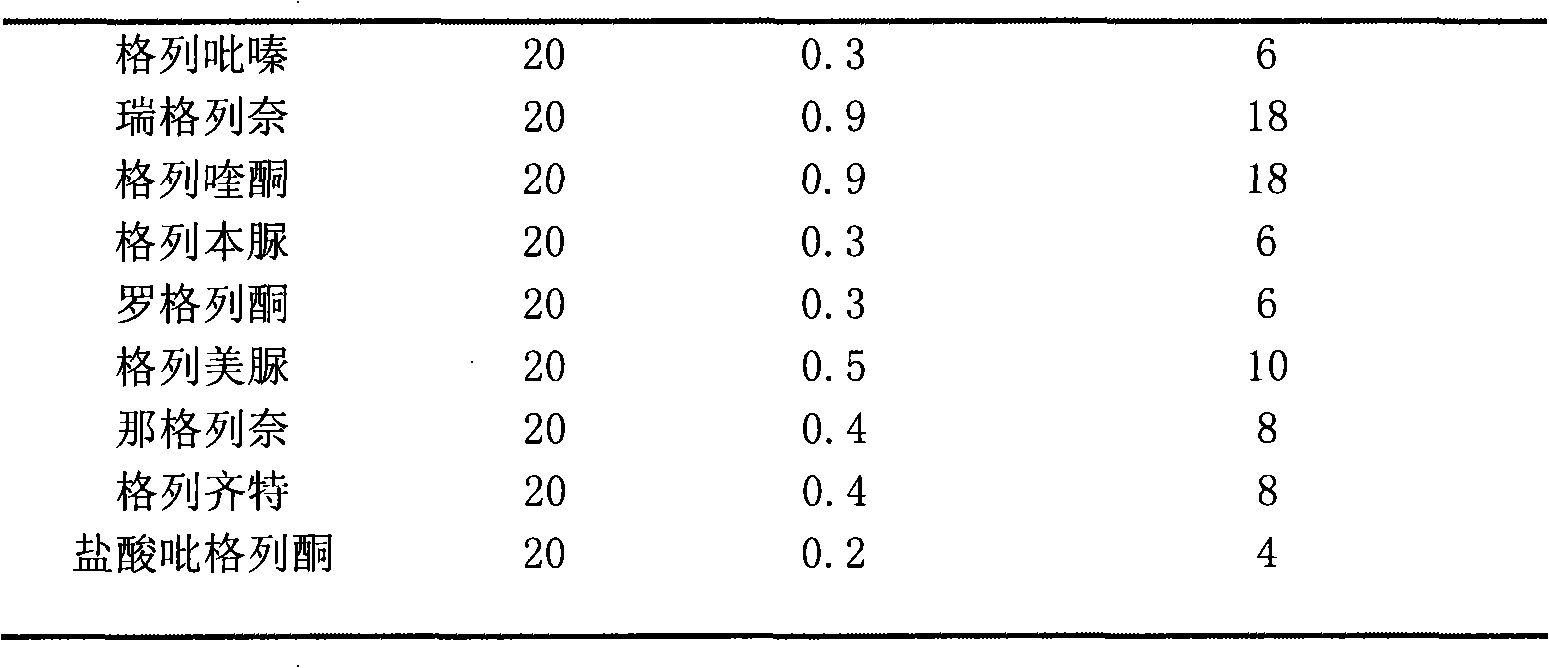
Gliquidone and rosiglitazone composition and preparation method thereof
PendingCN112245439AHigh single drug doseRich choiceMetabolism disorderSulfonylurea active ingredientsCelluloseIndividualized treatment
The present invention belongs to the field of medicine processing and discloses a gliquidone and rosiglitazone composition and a preparation method thereof. The gliquidone and rosiglitazone composition is composed of the following raw medicines in parts by weight: 30 mg-180 mg of gliquidone, 1 mg-8 mg of rosiglitazone, 10 mg-30 mg of carboxymethyl starch, 20 mg-50 mg of hydroxypropyl cellulose, 40mg-240 mg of lactose, and 1 mg-10 mg of magnesium stearate. The gliquidone and rosiglitazone are combined together, an adhesive, a diluent, a disintegrating agent and a lubricant are added as auxiliary materials, a part of the auxiliary materials are premixed, and finally dry tabletting or direct filling of capsules is carried out. Aiming at facts that diabetes is mainly treated by medicines currently domestically and individualized treatment is required, in order to avoid over-high dosage of a single medicine when a medicine effect of the single medicine is poor, the novel diabetes treatmentmedicine composition is provided to provide more choices for patients.
Owner:CHENGDU HENGRUI PHARMA
A kind of rosiglitazone gentisate and preparation method thereof
ActiveCN109053717BFast dissolution rateImprove apparent solubilityOrganic active ingredientsMetabolism disorderCholic acidBiochemical engineering
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Rosiglitazone saccharin salt and preparation method thereof
ActiveCN109053718AGood dissolution propertiesGreat tasteOrganic active ingredientsMetabolism disorderSolventSaccharin
The invention discloses rosiglitazone saccharin salt and a preparation method thereof. The rosiglitazone saccharin salt comprises rosiglitazone positive ions and saccharin negative ions of which a mole ratio is 1 to 1. In addition, the invention further discloses the preparation method for the rosiglitazone saccharin salt at the same time. The preparation method comprises the following steps: enabling rosiglitazone and saccharin to react in a solvent, through stirring, ultrasonic, volatilizing or cooling crystallization, to obtain the rosiglitazone saccharin salt. A type of brand-new rosiglitazone saccharin salt is provided. The rosiglitazone saccharin salt has apparent advantages in aspects of dissolution characters of drugs, improvement of mouthfeel and the like, and can be applied to apharmaceuticals industry, and the preparation method is simple in process, easy to control a crystallization process, good in repeatability, and suitable for industrialized production.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
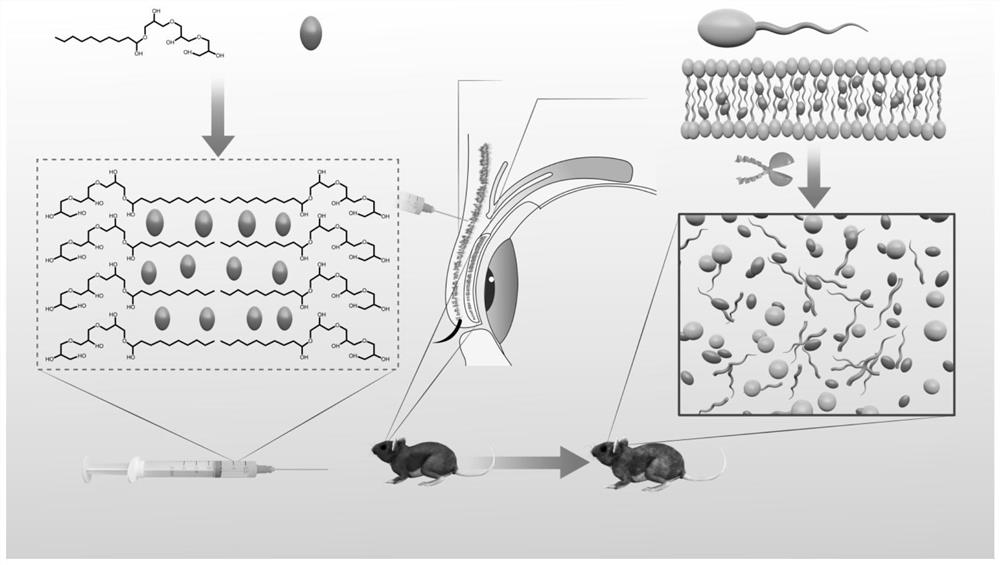
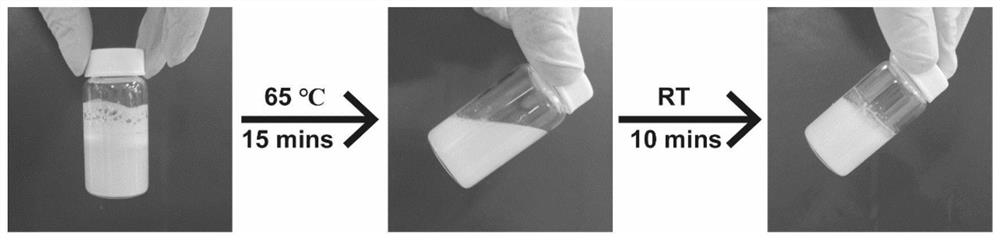
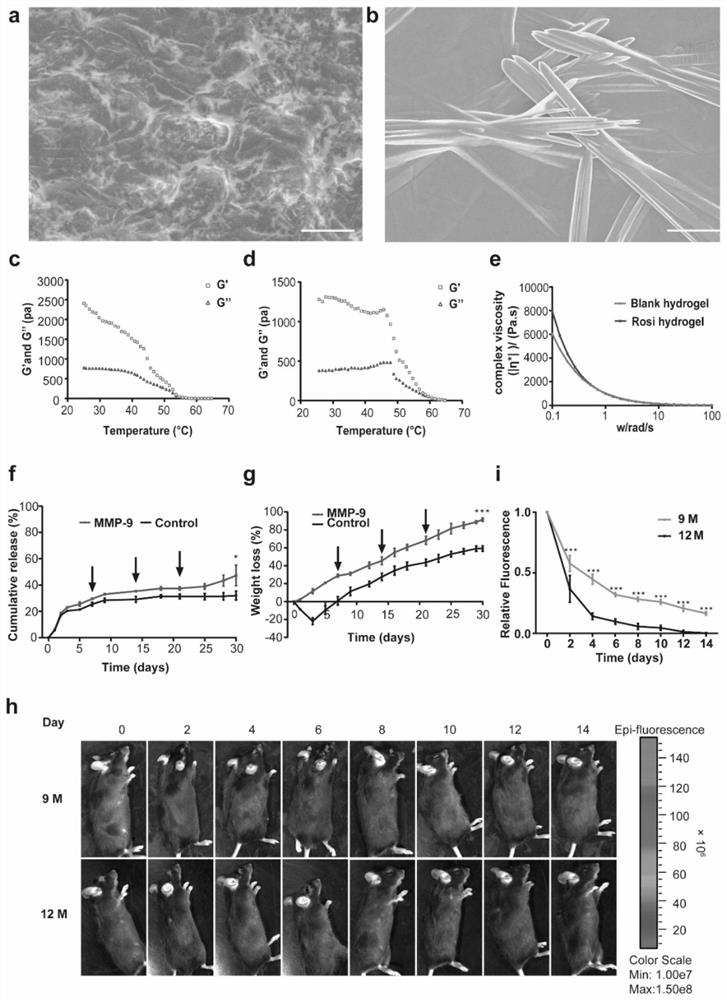
Injectable hydrogel system and preparation method thereof
The invention relates to the technical field of hydrogel, and particularly provides an injectable hydrogel system which takes injectable hydrogel as a continuous substrate phase, rosiglitazone is distributed in the continuous substrate phase, and the injectable hydrogel is obtained by self-assembling amphiphilic triglyceride monostearate in a solvent. The invention provides a preparation method ofthe injectable hydrogel system. The method comprises the following steps: heating a mixed solution containing rosiglitazone and amphiphilic triglyceride monostearate, and standing and cooling to obtain the injectable hydrogel system. The invention provides an application of the injectable hydrogel system in preparation of a product for treating meibomian gland dysfunction. The injectable hydrogelsystem prepared by the method has feasibility and high efficiency in prevention and treatment of age-related meibomian gland dysfunction.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
A kind of preparation method and application of phbv/rosiglitazone slow-release film
InactiveCN108743566BRelieve painImprove the success rate of surgeryOrganic active ingredientsSenses disorderOphthalmologyElectrospinning
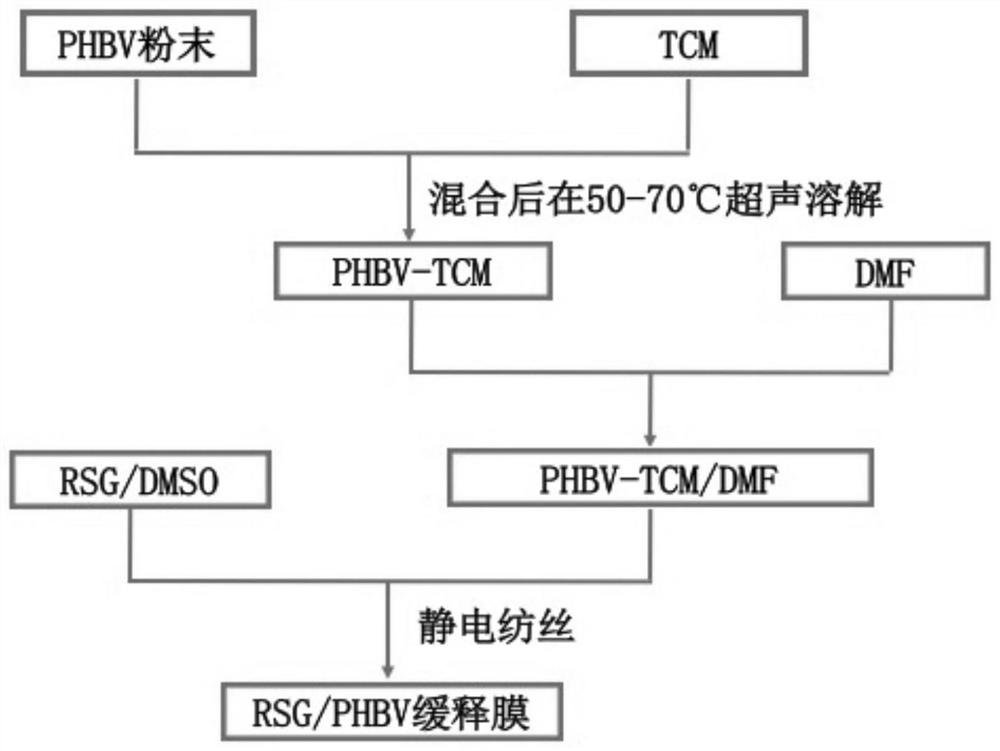
The invention discloses a preparation method and application of a PHBV / rosiglitazone sustained-release film, which belongs to the technical field of medicine. After mixing PHBV powder, chloroform and dimethylformamide, it is configured into a PHBV with a mass-volume ratio of 5%. ‑TCM / DMF silk solution for use. Rosiglitazone was dissolved in dimethyl sulfoxide solution to prepare RSG / DMSO solutions with different concentration gradients. Add it into the spinning solution, configure the spinning solution with different final concentrations, transfer it into a glass syringe, and perform electrospinning to form a rosiglitazone / PHBV slow-release film, which is used in anti-glaucoma filtration surgery. It is safe and It has very important clinical significance to effectively and durably prevent scar formation after glaucoma surgery and improve the long-term success rate of glaucoma surgery.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Medicine for preventing and treating diabetes and preparation method thereof
InactiveCN108218861AGood inhibitory effectLower blood sugar levels in ratsOrganic active ingredientsOrganic chemistryPositive controlProtein Tyrosine Phosphatase 1B
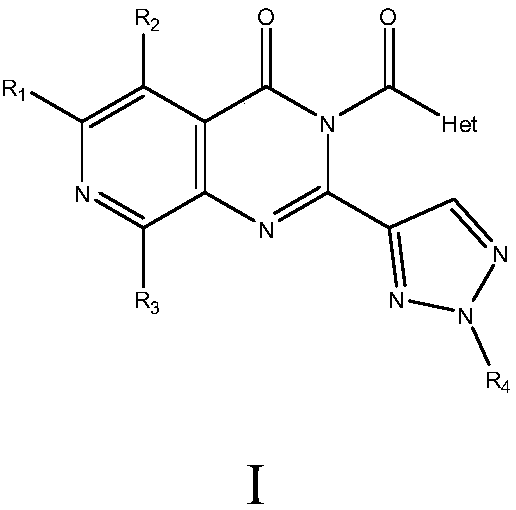
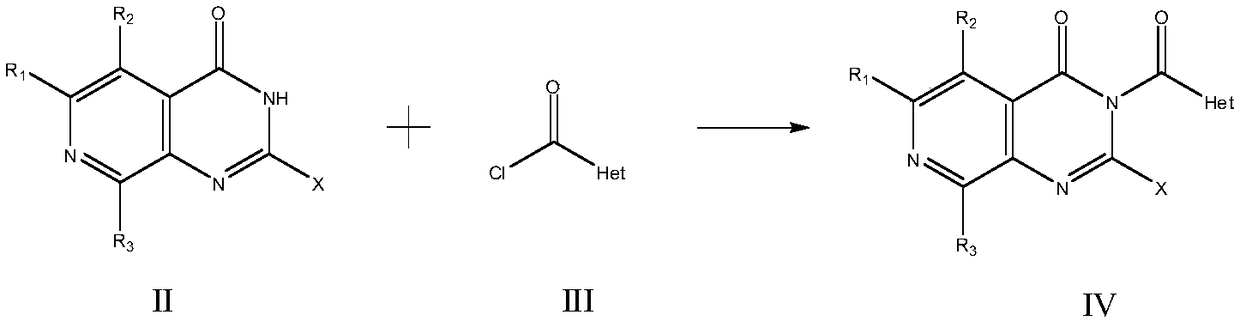
The invention relates to medicine with protein tyrosine phosphatase 1B(PTP1B) inhibition activity and application thereof. The compound is prepared from compound shown in a formula I or pharmaceuticalsalt and precursor of the compound. The compound disclosed by the invention shows an obvious inhibition effect on the PTP1B and can obviously reduce a rat blood sugar value in animal experiments; compared with positive control rosiglitazone, a sugar reducing effect of the compound is further improved; thus, the compound has an effect of remarkably controlling a blood sugar value of a diabetes ratand can be applied to preventing and treating diabetes, especially II type diabetes.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
Solid oral preparation containing rosiglitazone and cetirizine hydrochloride
InactiveCN102389427AImprove anti-allergicCorrect allergic complicationsOrganic active ingredientsMetabolism disorderCetirizine HydrochlorideCoated tablets
The invention discloses a solid oral preparation containing rosiglitazone and cetirizine hydrochloride. The solid oral preparation comprises 5 to 20mg of cetirizine hydrochloride and 1 to 12mg of rosiglitazone. The invention also discloses hard capsules, tablets, sustained-release coated tablets and pills made of the composition of rosiglitazone and cetirizine hydrochloride, as well as a preparation method thereof. The solid oral preparation has the advantages that: the rosiglitazone in the medicament can synergically improve the allergy resistance of cetirizine hydrochloride, is used for correcting allergic complication of diabetic patients, and is advantageous to reducing of forward risks of cardiovascular and cerebrovascular diseases of the diabetic patients.
Owner:CHENGDU HENGRUI PHARMA
Combination products containing limonoids and thiazolidinediones
The present invention relates to a compound containing limonoids (and pharmaceutically acceptable derivatives, esters, stereoisomers, salts or prodrugs thereof) and thiazolidinedione drugs (such as rosiglitazone, pioglitazone, etc. ) combination products. The present invention also relates to the use of the combined product in treating and / or preventing diseases related to diabetes.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
A kind of new rosiglitazone analogue and its preparation method and application
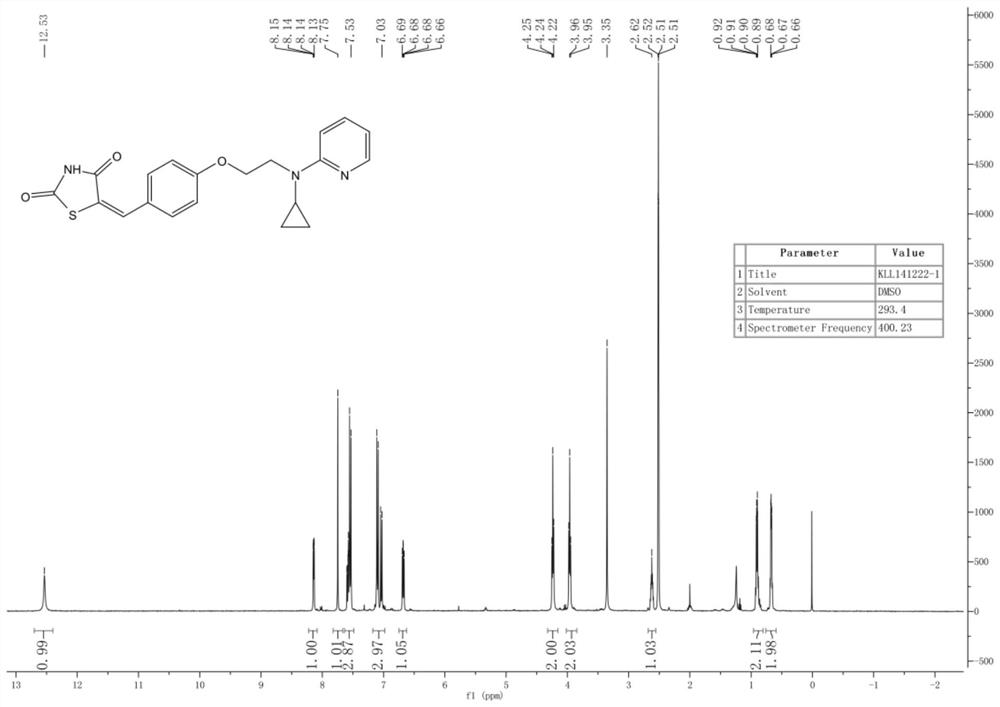
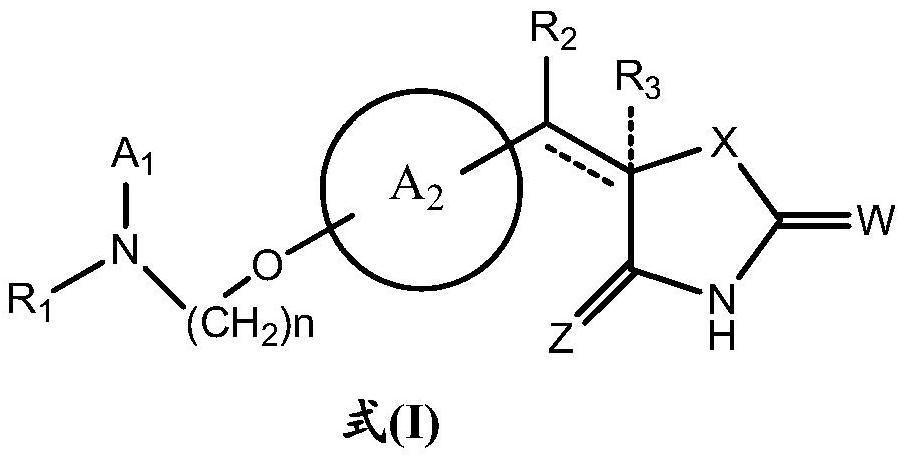
ActiveCN110627781BEasy to prepareImprove pharmacological activityOrganic active ingredientsOrganic chemistryDiabetes mellitusAryl
The invention relates to a rosiglitazone analogue, a preparation method and application thereof. Described compound has the structure shown in following formula (I), R 1 represents C that is unsubstituted or optionally substituted by 3‑10 Cycloalkyl: halogen, nitro, cyano, hydroxyl, mercapto, C 1‑6 Alkyl, C 1‑6 Alkoxy, halo C 1‑6 Alkyl; A 1 、A 2 The same or different, independently of each other represents C that is unsubstituted or optionally substituted by 6‑14 Aryl or 5‑14 membered heteroaryl: halogen, nitro, cyano, hydroxyl, mercapto, C 1‑6 Alkyl, C 1‑6 Alkoxy, halo C 1‑6 Alkyl; n is an integer of 0-5; R 2 means H or C 1‑6 Alkyl; R 3 Represents H; X, Z, W are the same or different, and independently represent S or O; represent a single bond or a double bond, and when it is a single bond, there is R 3 ; When double bond, R is absent 3 . The invention also provides its preparation method and its application in the preparation of medicines for treating or preventing diabetes.
Owner:北京安博睿达医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com