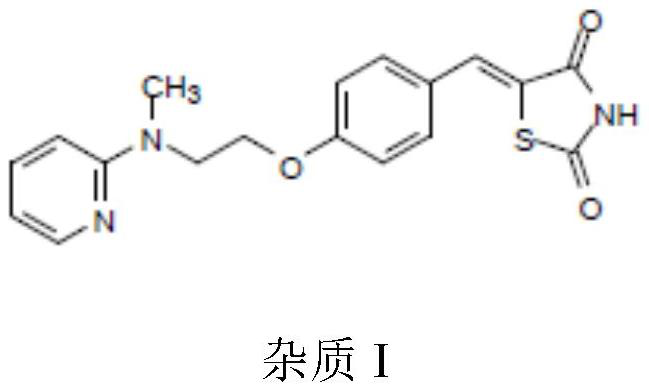
Impurity removal method of rosiglitazone hydrochloride
A technology of rosiglitazone hydrochloride and crude product, applied in the field of pharmaceutical synthesis, can solve the problems of impurity I being difficult to consume, unable to completely remove impurities and cost input, and achieve the effects of low cost, mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of method for removing impurities of rosiglitazone hydrochloride
[0027] The impurity-removing method of described rosiglitazone hydrochloride comprises the following steps:
[0028] Add 10 g of crude rosiglitazone hydrochloride (the content of impurity I is 0.7%), add 70 g of methanol, heat to 55 ° C to dissolve and clarify, then slowly add 0.1 g of magnesium powder for reaction, stir for 15 min, filter, and adjust the pH of the filtrate with hydrochloric acid when the filtrate is heated = 2 to 3, stirred for 30 minutes, then cooled the filtrate until a large amount of solids were precipitated, stirred at 0°C for 1 hour, filtered, rinsed, and dried to obtain high-purity rosiglitazone hydrochloride with a yield of 90% and an impurity I content of <0.01% or disappear.
Embodiment 2
[0029] The influence of embodiment 2 different solvents, ratio on the effect of removing impurities of rosiglitazone hydrochloride
[0030] With reference to the impurity removal method in Example 1, change the ratio of methanol or crude rosiglitazone hydrochloride to methanol, and remove impurities from the crude product of rosiglitazone hydrochloride (the content of impurity I is 0.7%). The effect of impurity removal is shown in Table 1 .
[0031] Table 1 The effect of different solvents and ratios on the removal of impurities of rosiglitazone hydrochloride
[0032] serial number solvent Crude product: solvent Impurity I content after impurity removal (%) Yield (%) 1 (Example 1) Methanol 1:7 <0.01
[0033] As can be seen from Table 1, after using methanol as solvent and the crude product of rosiglitazone hydrochloride to remove impurities according to a certain ratio, the content of impurity I is significantly reduced, and even can be completely rem...
Embodiment 3
[0034] The influence of embodiment 3 different reducing agents on the effect of removing impurities of rosiglitazone hydrochloride
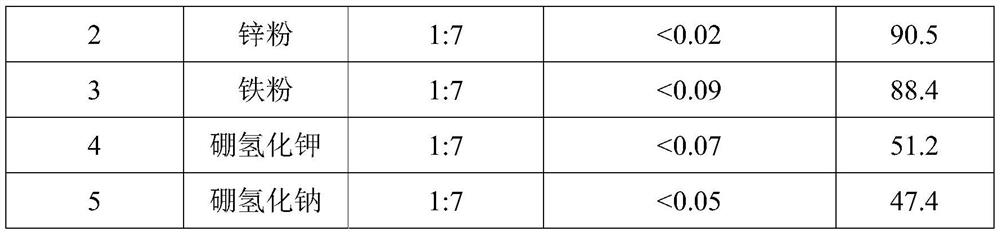
[0035] With reference to the impurity removal method in Example 1, the reducing agent was changed to remove impurities from the crude product of rosiglitazone hydrochloride (the content of impurity I was 0.7%). See Table 2 for the effect of impurity removal.
[0036] Table 2 The impurity removal effect of different reducing agents and addition amounts on rosiglitazone hydrochloride
[0037]
[0038]
[0039] As can be seen from Table 2, after using different reducing agents and the crude product of rosiglitazone hydrochloride to remove impurities according to a certain ratio, the content of impurity I is significantly reduced, and even can be completely removed, and the effect of removing impurities is very good; but when the reducing agent is boron During potassium hydride or sodium borohydride, although impurities can also be removed, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com