Medicine for preventing and treating diabetes and preparation method thereof
A prodrug and compound technology, applied in the field of medicine, can solve problems such as affecting insulin action, and achieve the effect of reducing blood sugar level in rats and improving hypoglycemic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
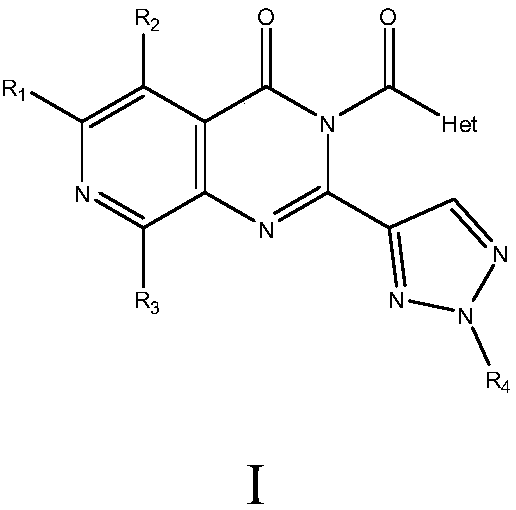
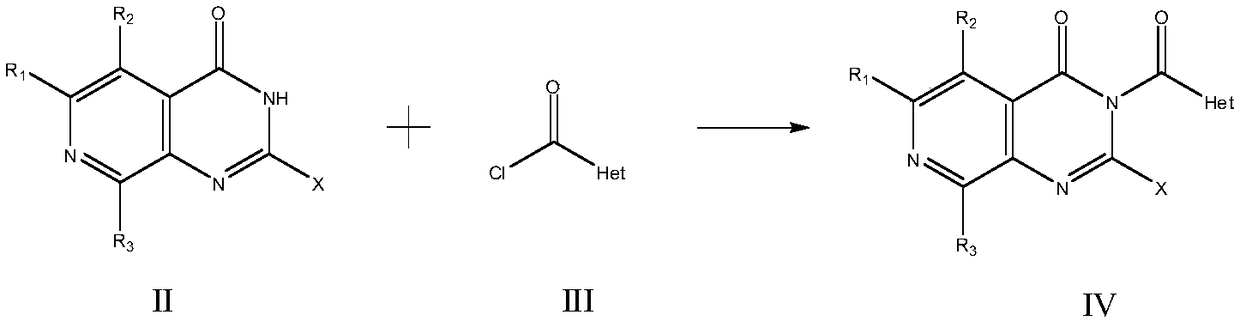
[0052] The preparation method of the compound of formula I of the present invention or its pharmaceutically acceptable salt comprises the following steps:
[0053] step one:
[0054]
[0055] This step involves reacting a compound of formula II with a compound of formula III and a base in an inert solvent to prepare a compound of formula IV.
[0056]Solvents used in this step can be alcohols such as methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, tert-butanol, isoamyl alcohol, octanol, cyclohexanol; aromatic hydrocarbons such as benzene , toluene or xylene; halogenated hydrocarbons such as chloroform, dichloromethane. The solvent is preferably an aromatic hydrocarbon, more preferably toluene.
[0057] The base used in this step can be a hydroxide such as sodium hydroxide, potassium hydroxide; a carbonate such as sodium carbonate, potassium carbonate; a bicarbonate such as sodium bicarbonate, potassium bicarbonate; an acetate such as Sodium acetate or p...
Embodiment 1
[0074] Example 1: 2-(2-methyl-2H-1,2,3-triazol-4-yl)-3-(pyridine-2-formyl)pyridin[3,4-d]pyrimidine-4( 3H)-Kone (Compound 1)
[0075]
[0076] Step 1: Add 3.08g (25.0mmol) of 2-pyridinecarboxylic acid, 100ml of toluene and DMF2ml in a 500ml there-necked flask with a stirring and reflux device, heat up and reflux, add dropwise a mixed solution of 10ml of thionyl chloride and 50ml of toluene, half Hours added, and then reflux constant temperature reaction for 4 hours. After the reaction is complete, stand still for half an hour to separate the liquid and remove the reaction impurities, then distill off excess thionyl chloride and toluene under reduced pressure to obtain 2-pyridinecarbonyl chloride, then add 50ml of toluene to dissolve it, and set aside. In a 500ml three-necked flask with a stirring and reflux device, add 4.48g (20.0mmol) of 2-bromopyridin[3,4-d]pyrimidin-4(3H)-one, 5.0g (50.0mmol) of potassium bicarbonate, A small amount of water and 100ml of toluene were st...
Embodiment 2
[0081] Example 2: 2-(2-methyl-2H-1,2,3-triazol-4-yl)-3-(piperidine-3-formyl)-8-(trifluoromethyl)pyridine[ 3,4-d]pyrimidin-4(3H)-one (Compound 2)
[0082]
[0083] According to the method of Example 1, replace 2-pyridinecarboxylic acid with piperidine-3-carboxylic acid, and use 2-bromo-8-(trifluoromethyl)-pyridin[3,4-d]pyrimidin-4(3H)-one Substitution of 2-bromopyridin[3,4-d]pyrimidin-4(3H)-one afforded the title compound as a white solid in 54% overall yield over two steps.
[0084] ESI-MS: 408.13[M+H] +
[0085] Elemental analysis: theoretical value / measured value, C(50.12 / 50.02), H(3.96 / 3.87), F(13.99 / 14.07), N(24.07 / 24.12), O(7.86 / 9.92)
[0086] 1 H NMR (400MHz, CDCl 3 )δ8.89(d,1H),8.14(s,1H),7.69(d,1H),3.63(s,3H),3.12(q,1H),2.86(q,1H),2.74(m,2H ), 2.47(s,1H), 2.06(s,1H), 1.86(m,1H), 1.64(m,1H), 1.56(m,1H), 1.42(m,1H).
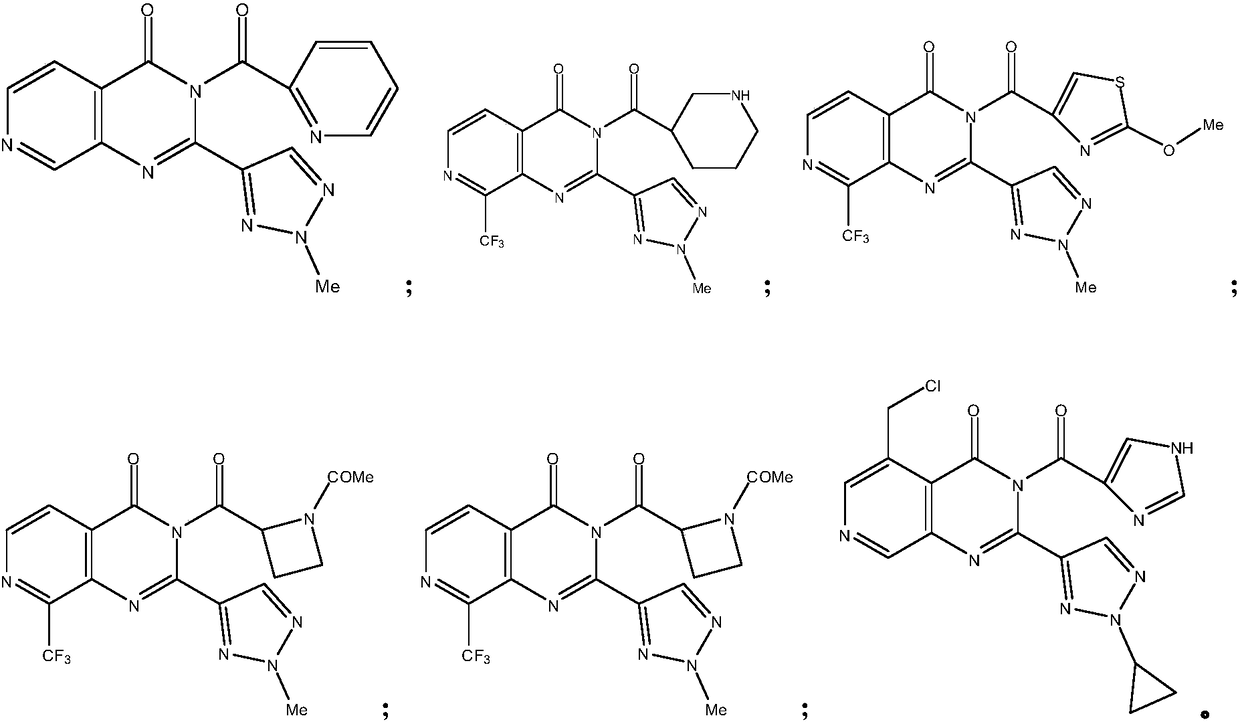
[0087] Following a similar method, the following compounds were synthesized:
[0088]
[0089]
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com