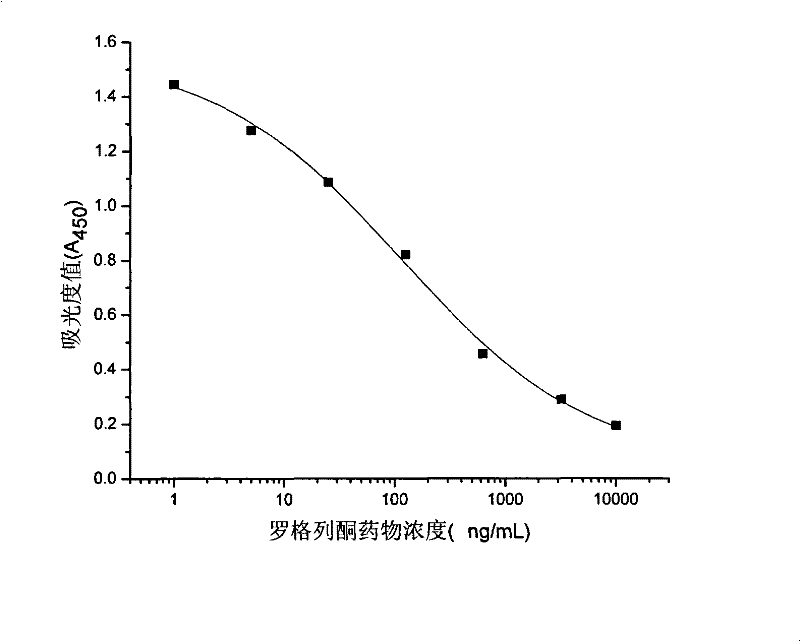
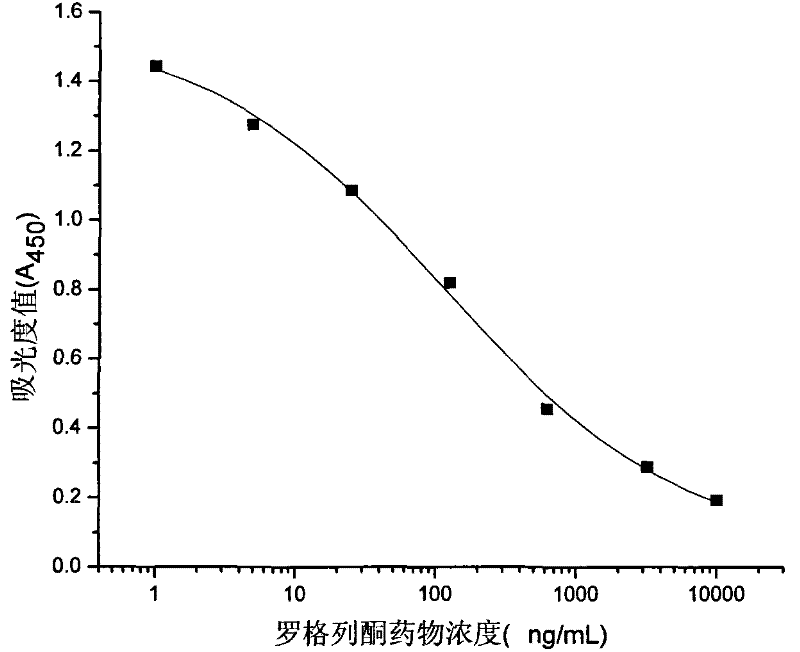
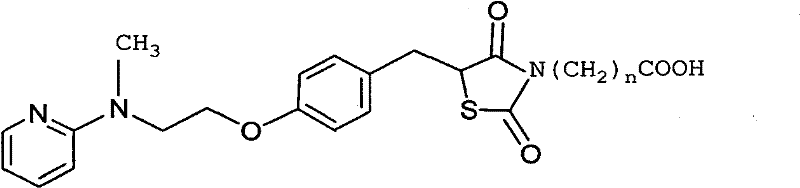
Rogridone hapten, artificial antigen and antibody as well as preparation method and application thereof
A technology of rosiglitazone and artificial antigen, which is applied in the field of preparation of artificial antigen and antibody, can solve the problems such as no rosiglitazone immunological detection method, and achieve the effect of high affinity, fast speed and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) Preparation of rosiglitazone hapten (n=1), as shown in the following reaction formula:
[0058]
[0059] Weigh 0.43g (1mmol) of rosiglitazone (purity: 98.5%) and 0.232g (2mmol) of sodium chloroacetate (analytically pure) and place it in a round-bottomed flask, and dissolve its mixture with 5ml of absolute ethanol , while adding 2mmol potassium carbonate, reflux and stir the reaction at 60°C, add 1mmol potassium iodide as a catalyst during the reaction, and reflux and stir for 4 hours. The reaction mixture was filtered to obtain a yellow-brown filtrate, which was concentrated to obtain a brown-yellow oily substance. The brown-yellow greasy substance was passed through a silica gel column for silica gel column chromatography, and the eluate of the chloroform / methanol elution phase with a volume ratio of 8:1 was collected, and the eluate was concentrated to dryness to obtain 0.09 g of rosiglitazone hapten.
[0060] (2) The preparation of rosiglitazone artificial an...
Embodiment 2
[0068] (1) Preparation of rosiglitazone hapten (n=6), as shown in the following reaction formula:
[0069]
[0070] Take by weighing 0.43g (1mmol) of rosiglitazone (purity: 98.5%) and 0.76g (5mmol) of chloroheptanoic acid (analytically pure) sodium and place it in a round bottom flask, and its mixture is washed with 5ml of absolute ethanol Dissolve, add 5mmol potassium carbonate at the same time, reflux and stir the reaction at 50°C, add 5mmol potassium iodide as a catalyst during the reaction, reflux and stir the reaction for 5 hours, then stop the reaction. The reaction mixture was filtered to obtain a yellow-brown filtrate, which was concentrated to obtain a brown-yellow oily substance. Pass through a silica gel column for silica gel column chromatography, collect the eluate from the chloroform / methanol elution phase with a volume ratio of 7:1, concentrate the eluate to dryness, and obtain 0.08 g of rosiglitazone hapten.
[0071] (2) The preparation of rosiglitazone art...
Embodiment 3
[0076] Example 3 Preparation of Rosiglitazone Hapten (n=1), R is Br, as shown in the following reaction formula:
[0077]
[0078] Weigh 0.43g (1mmol) of Rosiglitazone (purity: 98.5%) and 0.16g (1mmol) of sodium bromoacetate (analytically pure) and place in a round bottom flask, and its mixture is dissolved with 5ml of absolute ethanol , while adding 1mmol of potassium carbonate, reflux and stirring reaction at 70°C, adding 0.75mmol of potassium iodide as a catalyst during the reaction, reflux and stirring for 6 hours, then stop the reaction. The reaction mixture was filtered to obtain a yellow-brown filtrate, which was concentrated to obtain a brown-yellow oily substance. Pass through a silica gel column for silica gel column chromatography, collect the eluate from the chloroform / methanol elution phase with a volume ratio of 6:1, and concentrate the eluate to dryness to obtain 0.10 g of rosiglitazone hapten.
[0079] (2) The preparation of rosiglitazone artificial antigen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com