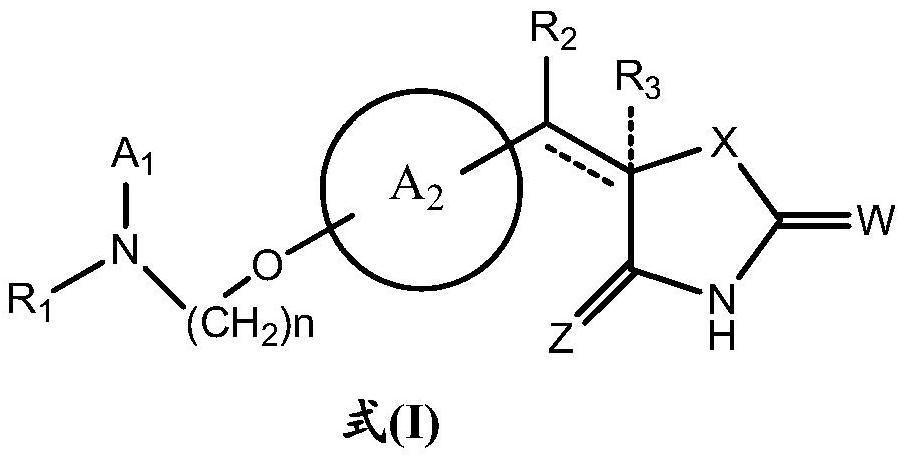
A kind of new rosiglitazone analogue and its preparation method and application
A technology of reaction and scheme, applied in the field of compound and its preparation, to achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
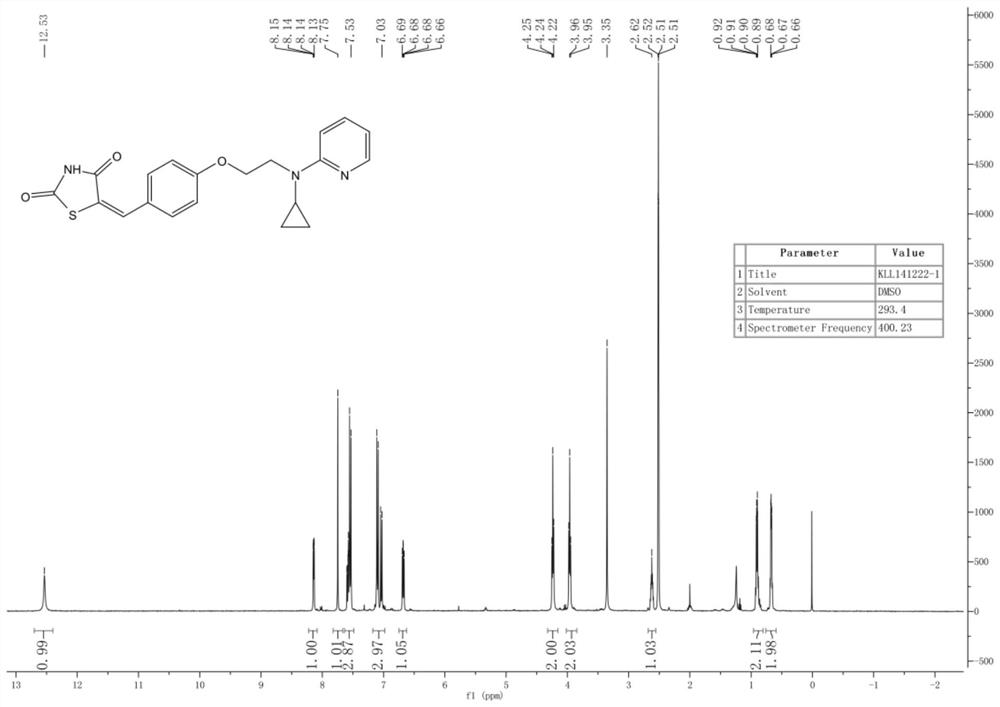
[0098] Example 1: (E)-5-(4-(2-(cyclopropyl(pyridin-2-yl)amino)ethoxy)benzylidene)thiazolidine-2,4-dione (compound II-1 )
[0099]
[0100] step 1:
[0101]
[0102] Add A1 (50g, 0.3286mol, 1eq) and 1,2-dibromoethane (500g, double as solvent and raw material, excess) in a 500ml single-necked flask, and add potassium carbonate (63.0g, 0.4558mol, 1.3eq), the temperature was raised to 120°C, and the reaction was carried out overnight, monitored by TLC until the reaction was complete. Stop heating, cool down, evaporate 1,2-dibromoethane under reduced pressure with an oil pump at 70°C, evaporate to dryness, add water to dissolve, extract with 800ml dichloromethane, dry over anhydrous magnesium sulfate, concentrate by suction filtration, pack 250g silica gel column, wet The method was used to load the sample, and 70 g of slightly pure light yellow oil (solid after standing for a long time) was obtained, and the yield was 82.35%.
[0103] Step 2:
[0104]
[0105] Add A2 (7...
Embodiment 2
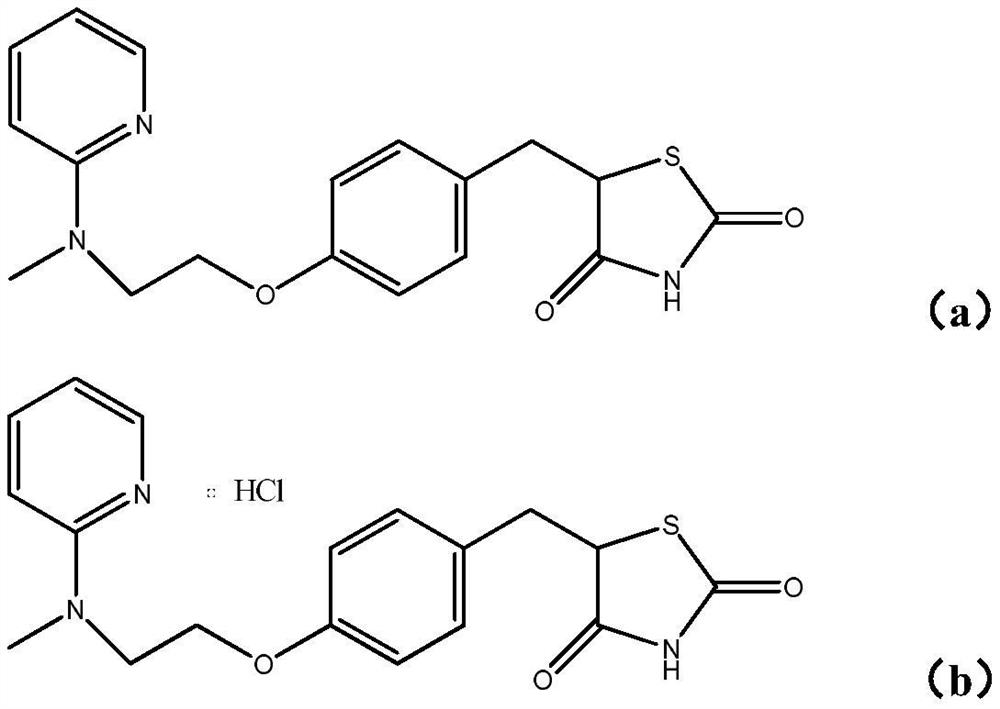
[0129] Example 2: 5-(4-(2-cyclopropyl(pyridin-2-yl)amino)ethoxy)benzylthiazolidine-2,4-dione (compound I-1)
[0130]
[0131] On the basis of Example 1, step 9 is further included, and the product A9 is further reduced using palladium carbon catalytic hydrogenation. MS (m / z): 384.1 (M+H).
Embodiment 3
[0132] Example 3: 5-(4-(2-cyclopropyl(pyrimidin-2-yl)amino)ethoxy)benzylthiazolidine-2,4-dione (compound 1-2)
[0133]
[0134] The preparation method is the same as in Example 2, except that Step 4 and Step 5 are omitted, and the raw material in Step 3 is 2-bromopyrimidine. MS (m / z): 386.1 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com