Solid oral preparation containing rosiglitazone and cetirizine hydrochloride
A technology of cetirizine hydrochloride and oral preparation, which is applied in the field of preparations containing rosiglitazone and cetirizine hydrochloride to achieve the effects of improving anti-allergic effect, reducing long-term risk and correcting allergic complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: contain the solid oral preparation of rosiglitazone and cetirizine hydrochloride, it is made up of the component of following weight ratio:
[0043] Cetirizine Hydrochloride 10mg
[0044] Rosiglitazone 4mg
[0045] Appropriate amount of accessories.
[0046] Wherein, described auxiliary material is:
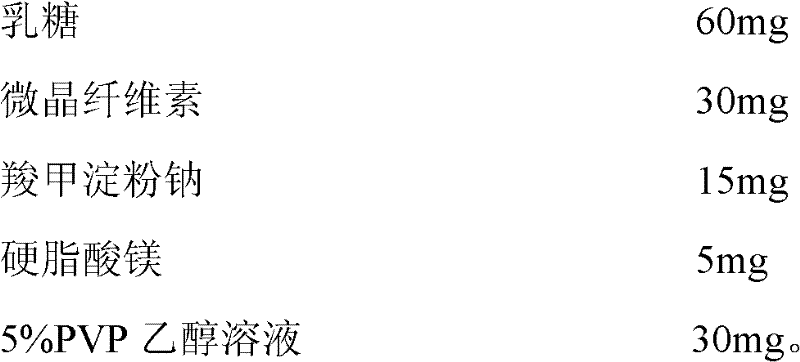
[0047]
[0048] The preparation method of the solid oral preparation containing rosiglitazone and cetirizine hydrochloride is:
[0049] 1. Pass 4 mg of the main drug rosiglitazone and 10 mg of cetirizine hydrochloride through a 100-mesh sieve, and 60 mg of lactose, 30 mg of microcrystalline cellulose and 15 mg of sodium carboxymethyl starch through a 80-mesh sieve, and mix well with the main drug ;
[0050] 2. Add 30 mg of 5% PVP ethanol solution, mix until dry and wet, and granulate with a 16-24 mesh sieve;
[0051] 3. Dry until the moisture content is below 5%, add 5 mg of magnesium stearate, mix evenly, pack into capsules to obtain the hard capsule...
Embodiment 2
[0053] A solid oral preparation containing rosiglitazone and cetirizine hydrochloride, which consists of components in the following weight ratios:
[0054] Cetirizine Hydrochloride 5mg
[0055] Rosiglitazone 12mg
[0056] Appropriate amount of accessories.
[0057] Wherein, described auxiliary material is:
[0058]
[0059] The preparation method of the solid oral preparation containing rosiglitazone and cetirizine hydrochloride is:
[0060] 1. Pass 12 mg of the main drug rosiglitazone and 5 mg of cetirizine hydrochloride through a 100-mesh sieve, 60 mg of lactose, 30 mg of microcrystalline cellulose and 15 mg of sodium carboxymethyl starch through a 80-mesh sieve, and mix well with the main drug ;
[0061] 2. Add 30 mg of 5% PVP ethanol solution, mix until dry and wet, and granulate with a 16-24 mesh sieve;
[0062] 3. Dry until the water content is below 5%, add 5 mg of magnesium stearate, mix evenly, pack into capsules to obtain the hard capsules of solid oral prep...
Embodiment 3
[0064] A solid oral preparation containing rosiglitazone and cetirizine hydrochloride, which consists of components in the following weight ratios:
[0065] Cetirizine Hydrochloride 20mg
[0066] Rosiglitazone 1mg
[0067] Appropriate amount of accessories.
[0068] Wherein, described auxiliary material is:
[0069]
[0070] The preparation method of the solid oral preparation containing rosiglitazone and cetirizine hydrochloride is:
[0071]1. Pass the main drug rosiglitazone 1 mg and cetirizine hydrochloride 20 mg through a 100-mesh sieve, the excipients lactose 60 mg, microcrystalline cellulose 30 mg and carboxymethyl starch sodium 15 mg through a 80-mesh sieve, and mix with the main drug evenly ;
[0072] 2. Add 30 mg of 5% PVP ethanol solution, mix until dry and wet, and granulate with a 16-24 mesh sieve;
[0073] 3. Dry until the moisture content is below 5%, add 5 mg of magnesium stearate, mix evenly, pack into capsules to obtain the hard capsules of solid oral ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com