Medicine composition for treating polycystic kidney disease and use thereof
A composition and medicine technology, applied in the field of pharmaceutical compositions and their applications and pharmaceutical preparations containing the same, can solve the problems of liver damage, increased incidence of heart failure, water and sodium retention and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
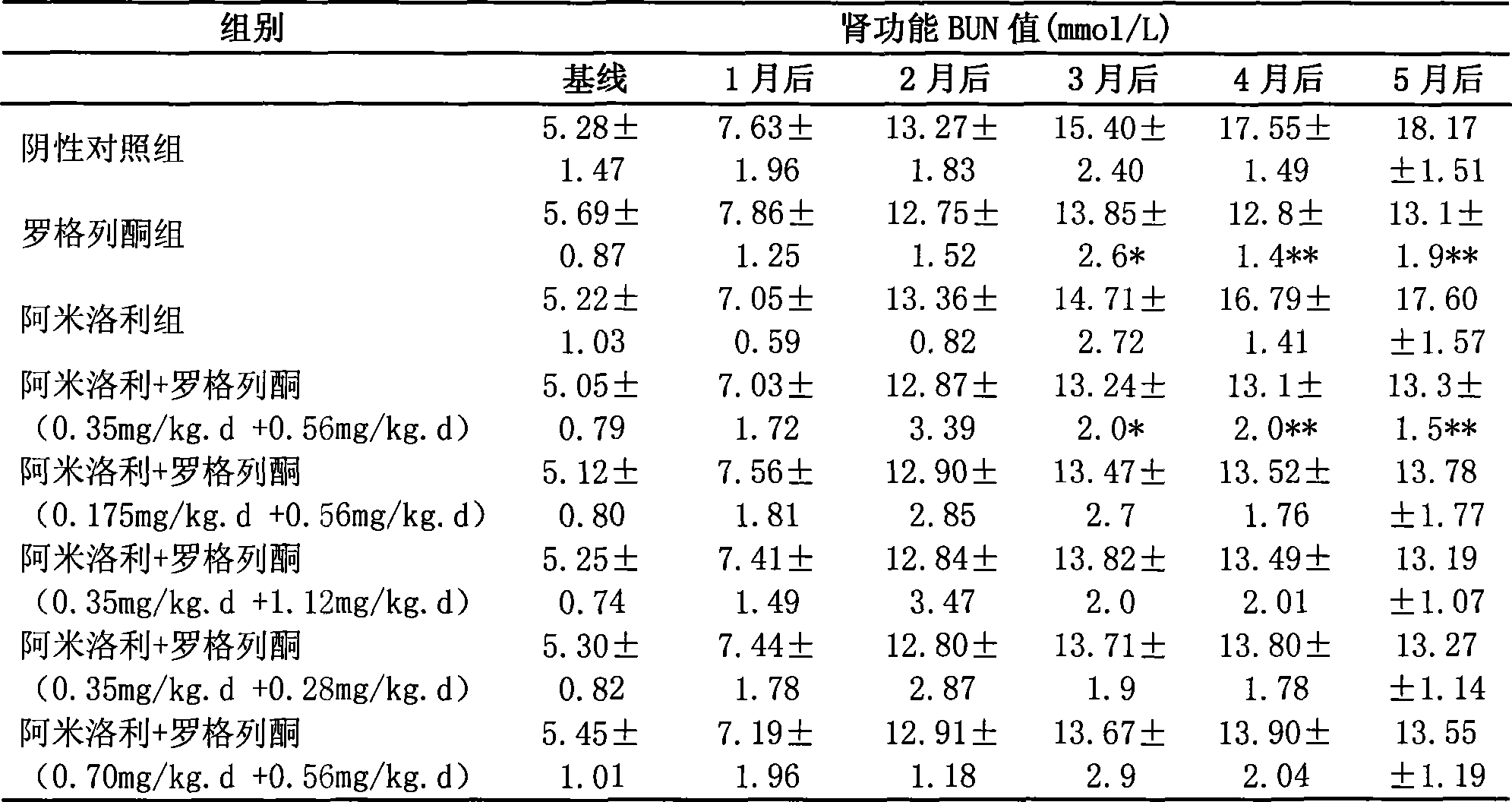
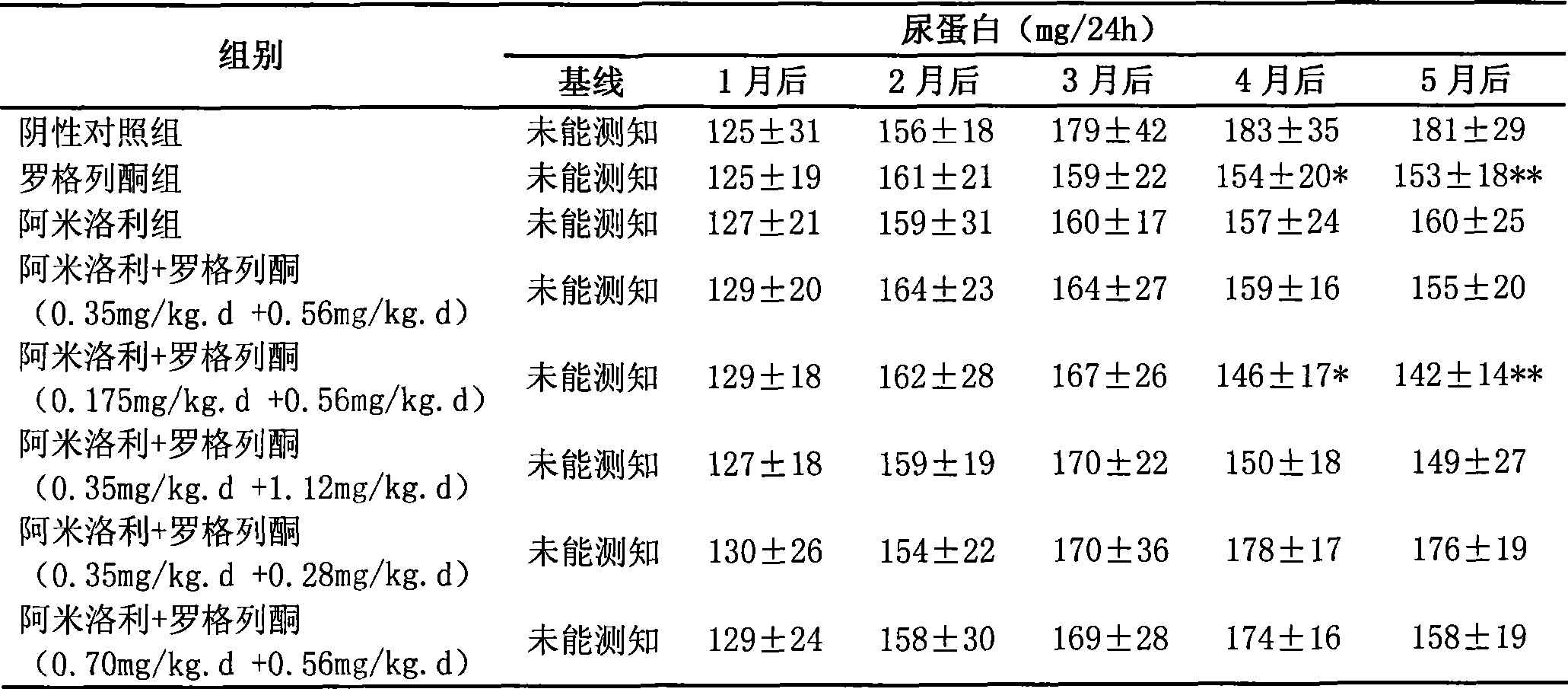
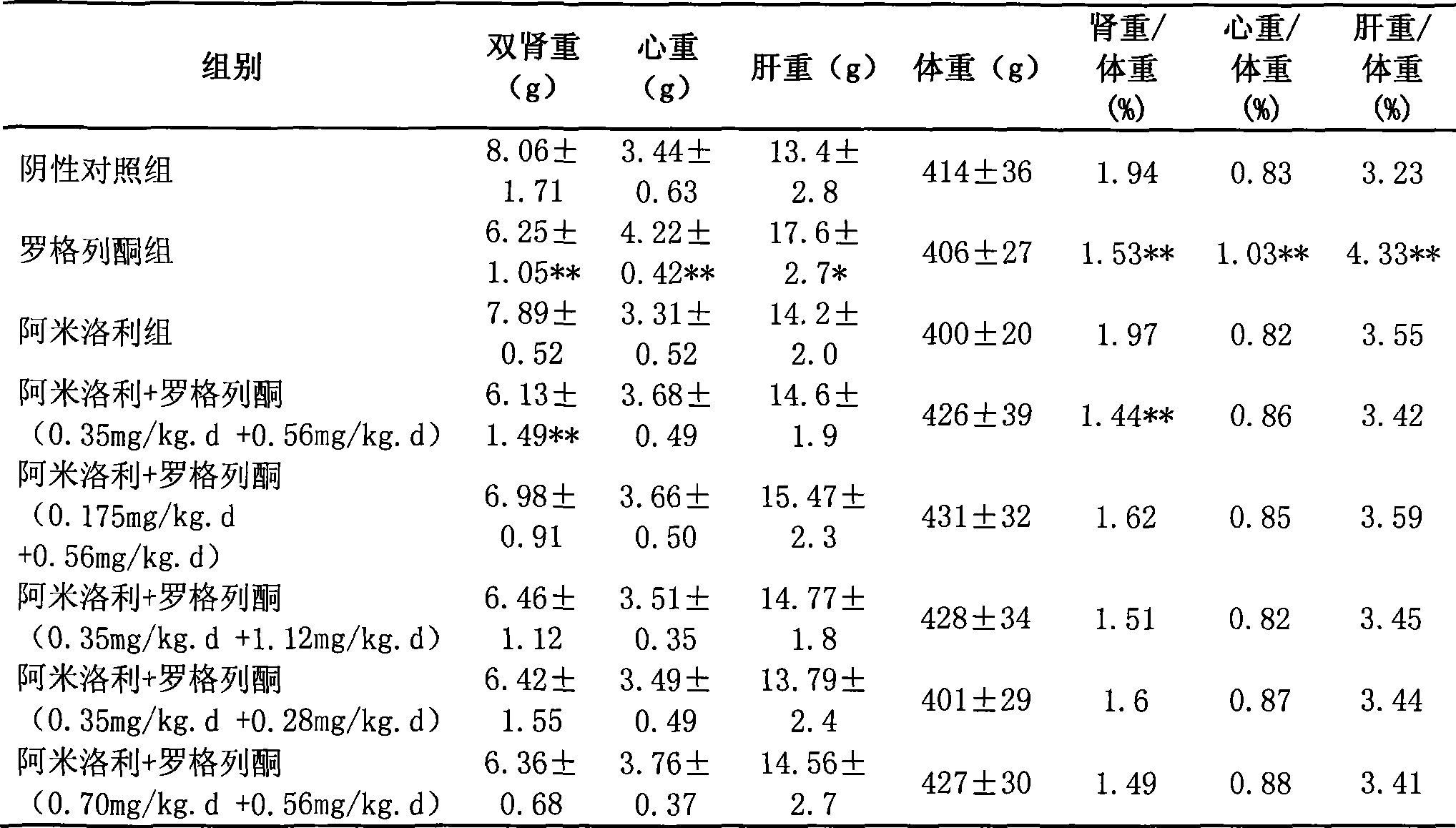
[0043] To observe the renal protective effects and side effects of long-term application of rosiglitazone, amiloride and their combination on ADPKD animal model Han: SPRD rats.
[0044] Experimental materials and methods
[0045] ADPKD animal model Han: SPRD rats were imported from the Mayo Clinic Medical Center in the United States and have been bred stably in the Experimental Animal Center of the Second Military Medical University for nearly 7 years. The inventor took Han:SPRD male 3-week-old heterozygous cy / + rats as intervention objects, and set up negative control group, rosiglitazone group (0.56mg / kg.d), amiloride group (0.35mg / kg .d) and 5 combined medication groups: amiloride+rosiglitazone (0.35mg / kg.d+0.56mg / kg.d), amiloride+rosiglitazone (0.175mg / kg.d +0.56mg / kg.d), amiloride+rosiglitazone (0.35mg / kg.d+1.12mg / kg.d), amiloride+rosiglitazone (0.35mg / kg.d +0.28mg / kg.d), amiloride+rosiglitazone (0.70mg / kg.d+0.56mg / kg.d). Gastrointestinal administration for 5 months, o...
Embodiment 2
[0064] Embodiment 2: Compound (amiloride hydrochloride+rosiglitazone maleate) solution
[0065] Prescription: Amiloride Hydrochloride 2.5g
[0066] Rosiglitazone Maleate 5g
[0067] Sodium Lauryl Sulfate 0.1g
[0068] Distilled water appropriate amount 10000ml
[0069] Process: Take the above-mentioned amiloride hydrochloride, rosiglitazone maleate and sodium lauryl sulfate, add 1000ml of distilled water to dissolve, then add 10000ml of distilled water to obtain.
Embodiment 3
[0070] Embodiment 3: Compound (amiloride hydrochloride + rosiglitazone maleate) syrup
[0071] Prescription: Amiloride Hydrochloride 1g
[0072] Rosiglitazone Maleate 2g
[0073] Distilled water 1500ml
[0074]Add simple syrup to 10000ml
[0075] Process: Dissolve amiloride hydrochloride and rosiglitazone maleate in distilled water, add simple syrup to the full amount, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com