Artemisia plant pollen allergen combination, application and kit
A technology for pollen allergies and Artemisia plants, applied in the biological field, can solve the problems of insufficient analysis of allergic asthma and insufficient analysis of allergic asthma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
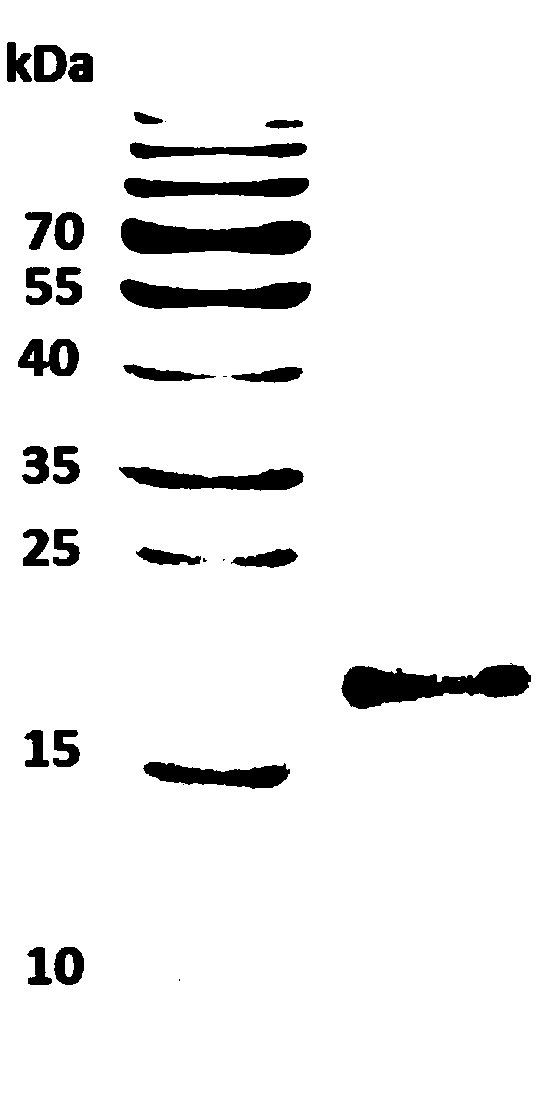
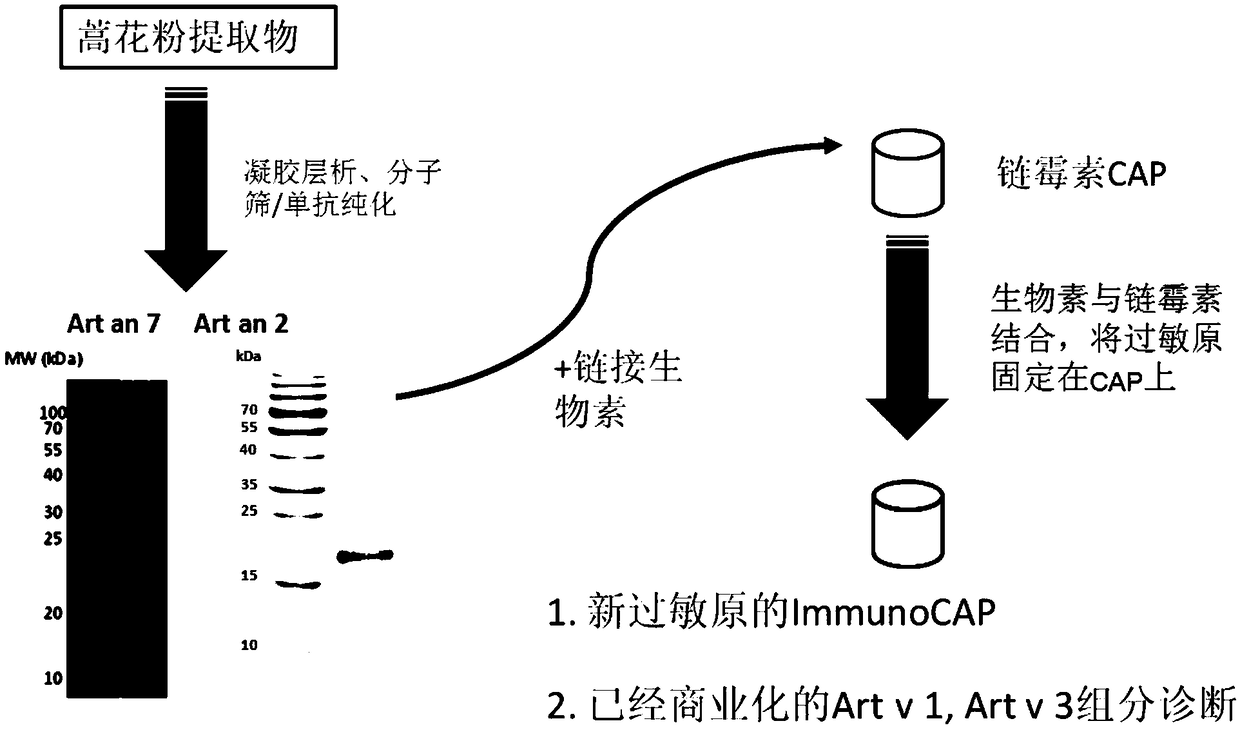
[0037] Art ar 2 separation and purification, the application of a mouse monoclonal antibody C9-C1 immunoaffinity separation.
[0038] The source of the monoclonal antibody was to immunize 5 mice with 100 μg Artemisia mugwort pollen protein extract (SCXK(HU) 2012-002, China), and inject 50 μg Artemisia protein extract every 7-14 days for a total of six times. According to Western blot and ELISA screening, 3 monoclonal antibodies were obtained, 2 of which bound to Art v 3, and one bound to a protein with a molecular weight of about 16kDa. Take an appropriate amount of CNBr-activated Sepharose 4 Fast Flow (GE healthcare) and add 4 times the volume of cold 1mM HCl, stir and remove the liquid, ddH 2 After washing, mix well with C9-C1 monoclonal antibody dialyzed into coupling buffer, couple overnight at 4°C, measure the concentration of uncoupled protein in the solution to determine the coupling efficiency, and wash with at least 5 times the volume of coupling buffer To remove exc...
Embodiment 2
[0040] The detection of Art v 1 and Art v 3 directly refers to the ImmnuoCAP product manual.
[0041] ImmunoCAP detection of natural allergens Art ar 2 and Art an 7: After the purified natural allergens Art ar2 and Art an 7 were linked with biotin, they were added to streptavidin-conjugated ImmunoCAP (O212) at 37°C at an amount of 0.5 μg / CAP. After incubation for 1 hour, the residual liquid was sucked off for later use (Erwin et al., 2005).
[0042] The specific IgE antibody content was determined by ImmunoCAP (Thermo Fisher, USA), and the detected Artemisia argyi allergen was the extract of Artemisia argyi (w6) and allergen components nArt v 1 (w231), nArt v 3 (w233), nArt ar 2 And nArt an 7, the amount of serum is 40μl, sIgE ≥ 0.35kUA / L is considered positive. The operation process and method, standard curve and positive quality control refer to the ImmnuoCAP 100 product manual. See the test process image 3 . Case information and IgE in vitro test results are shown in T...
Embodiment 3
[0052] At present, there are 7 allergen components of Artemisia pollen (Art v 1–Art v 7), and there are only 2 commercial components for ImmunoCAP detection by ThermoFisher, Sweden: Art v1 and Art v 2. The sum of the measured values of the four allergen components measured by the present invention has a very high correlation (r=0.92, see Figure 4 ). Only a small number of sera lacked the corresponding components, but most of these cases had mild allergic symptoms. These four components are therefore fully representative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com