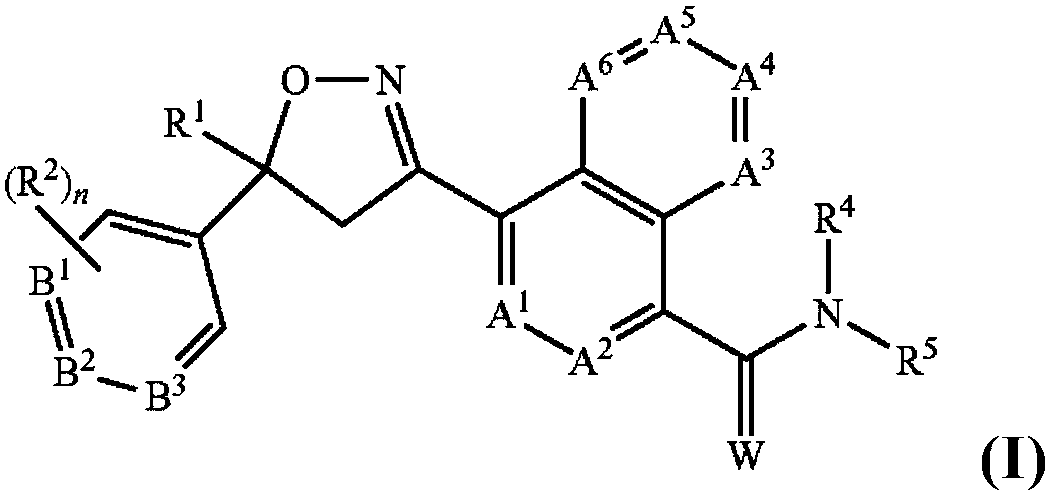
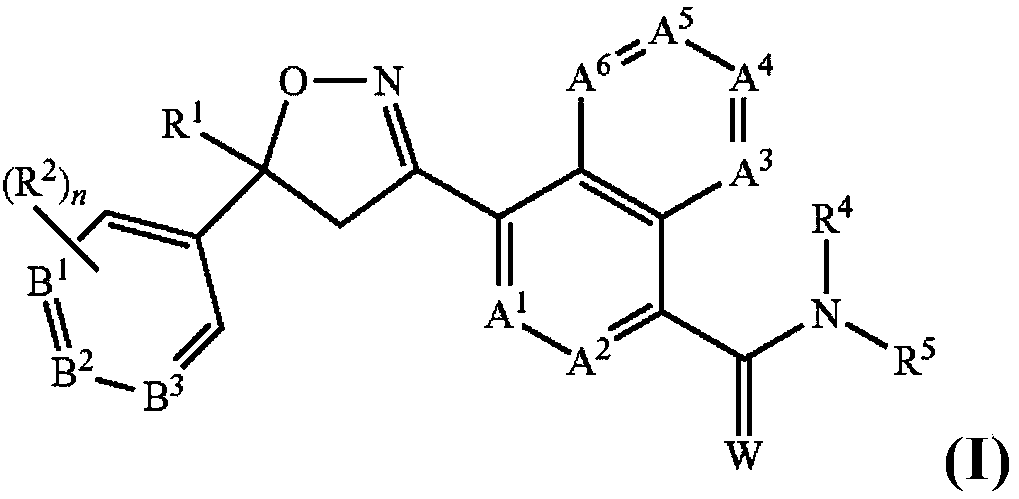
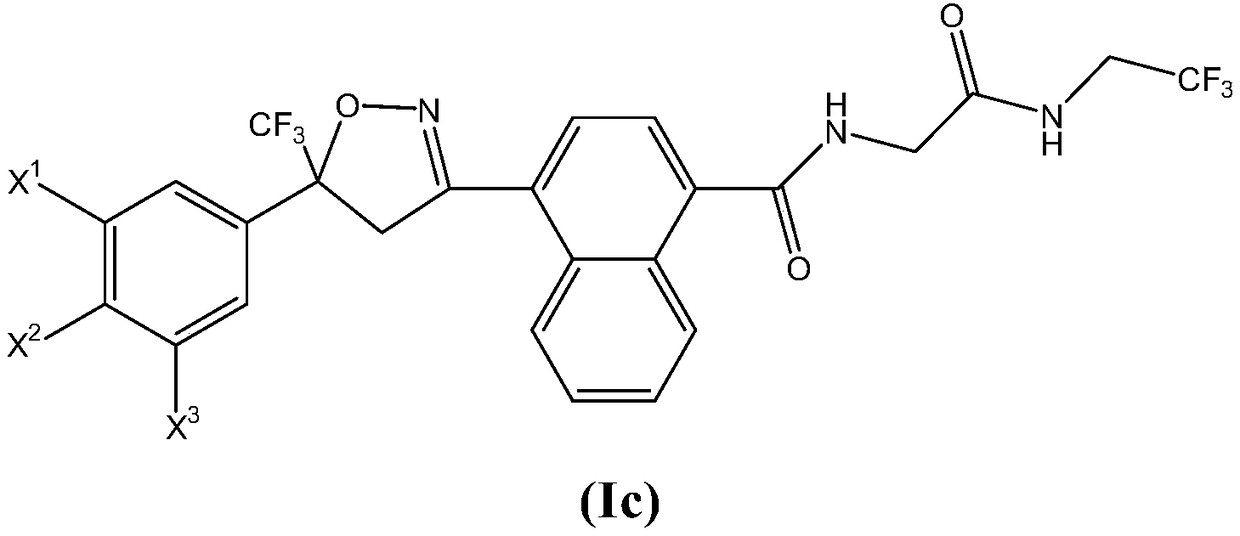
Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof
A technology of isoxazoline and compounds, applied in the field of isoxazoline compounds, can solve problems such as economic loss, impact on animal nutrition, impact on companion animals and poultry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1594] Preparation method of compound of formula (Id)
[1595] Approved from US 7964204, US 8410153, US 8546,618, US 8217180, US 8 546613, US7662972, US 8466115, US 8383659, US 8853186, US 8618126, US 2014 / 0371464, US 2015 / 0291612 and WO 2014 / 090918 ( They are all incorporated herein by reference in their entirety by methods improved from those described in), which can prepare the compound of formula (Id) and the intermediates used in the method for preparing the compound.
[1596] In one embodiment, the compound of the present invention can be prepared according to the general method shown in the following scheme 1, wherein Y, Q, X 1 And X 2 As defined above with respect to formula (Id), W is Cl, Br or I, and R is an alkyl group.
[1597] plan 1
[1598]
[1599] This general method is described in, for example, US 8,546,618 to prepare compounds where Y is Y-2. Using appropriately substituted compounds of formula Id-1 and Id-4, a variety of compounds of formula (Id) can be prepared...
Embodiment
[1898] The following non-limiting examples further describe the invention, which further illustrate the invention, and they are not intended and should not be construed as limiting the scope of the invention.
[1899] Synthesis Example
[1900] Synthesis Example 1: Synthesis of (S)-Ie
[1901] The compound of formula (S)-Ie of the present invention is prepared according to Scheme 2 below. Compound 2-1 is described in US 7,951,828 Bl (incorporated herein by reference). The preparation of compounds 2-4 is described in US 8,217,180 B2 and US 8,546,618 B2 (both of which are incorporated herein by reference in their entirety). According to operations described in, for example, US 9,126,995 B2, WO 2011 / 104089, and US2014 / 0206633 (all incorporated herein by reference), a cinchona alkaloid-based chiral phase transfer catalyst similar to 2-6 was prepared. In addition, Matoba et al., Angew. Chem. 2010, 122, 5898-5902 describe the use of these catalysts for the preparation of enantiomerical...
preparation Embodiment 5
[1928] Compound of formula (Ia) 30% (w / v)
[1929] Ethanol 9% (w / v)
[1930] PEG 400 QS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com