CD20-binding single domain antibody
A single-domain antibody, binding agent technology, applied in the direction of antibodies, antibody medical components, antibody mimetics/scaffolds, etc., can solve problems such as toxicity and side effects, rituximab treatment response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0412] Example 1. Construction and evaluation of human CD20-specific VHH
[0413] Cloning of human CD20 gene
[0414] Human CD20 was amplified from Orfeome v5.1 collection (ID 11051 ) using forward primer 5'-GATAAGATCTCAGGCGGATCCACAACACCCAGAAATTCAG (O-7954) and reverse primer 5'-GGTTTTTCTCTAGATCAAGGAGAGCTGTCATTTTCTATTGG (O-7956). The amplified product was cut with BglII and Xbal and ligated into the mammalian expression vector pMet7. The plasmid was used for transient transfection of Hek293T cells and generation of CHO-K1 clones stably expressing human CD20.
[0415] Isolation of antigen-specific VHH
[0416] A VHH library was constructed and three consecutive rounds of panning (in solution) were performed on stably transfected CHO-K1 cells expressing human CD20. Parallel panning was performed on parental (untransfected CHO-K1 ) cells to serve as a negative control in order to calculate CD20-specific phage enrichment. Enrichment of antigen-specific phage was assessed aft...
Embodiment 2
[0447] Example 2. Construction and evaluation of mouse CD20-specific VHH
[0448] Cloning of mouse CD20 gene
[0449] Mouse CD20 was purchased from Imagenes, catalog number IRAVP968C1280D, and amplified with the forward primer 5'-gataagatctcaGGCGGATCCAGTGGACCTTTCCCAGCAGAGC (0-7962) and the reverse primer 5'-GGTTTTTCTCTAGATCAAGGAGCGATCTCATTTCCACTG (0-7964). The amplified product was cut with BglII and Xbal and ligated into the mammalian expression vector pMet7. The plasmid was used for transient transfection of Hek293T cells and generation of CHO-K1 clones stably expressing mouse CD20.
[0450] Isolation of antigen-specific VHH
[0451] The VHH library was constructed from peripheral blood lymphocytes (PBL). Specifically, total RNA from PBLs was used as template for first-strand cDNA synthesis with oligo(dT) primers. Using this cDNA, the VHH coding sequence was amplified by PCR, digested with PstI and NotI, and cloned into the PstI and NotI sites of the phagemid vector pM...
Embodiment 3
[0486] Example 3. Functional assessment of human CD20 and / or mouse CD20-specific VHHs
[0487] Demonstration of specific binding of VHHs by FACS
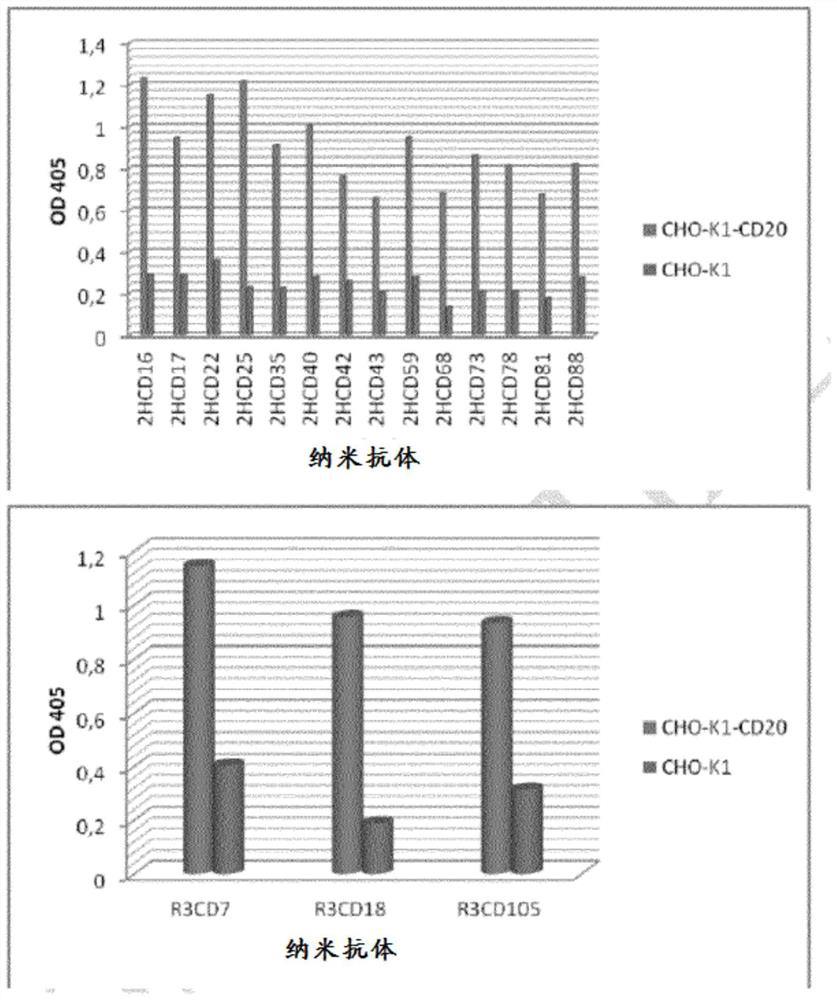
[0488] To determine the binding activity of human or mouse CD20-specific VHHs, FACS analysis was performed. Specifically, CHO-K1 cells stably expressing human or mouse CD20 and CHO-K1 cells were incubated with 5 μg / ml of VHH produced according to the present application. A FITC-labeled monoclonal anti-his antibody (Genscript, Cat. A01620) was used as a secondary stain. FACS analysis was performed on a FacsCalibur flow cytometer (Becton Dickinson). like Figure 8 As shown, all VHHs against human CD20 showed specific binding to human CD20. Similarly, all VHHs against mouse CD20 also showed specific binding to mouse CD20 ( Figure 9 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com