Preparation method, application and preparation primer of influenza virus HA fusion peptide and helix A protein
A technology of influenza virus and fusion peptide, applied in the direction of virus peptide, fusion peptide, chemical instruments and methods, etc., to achieve good immunogenicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
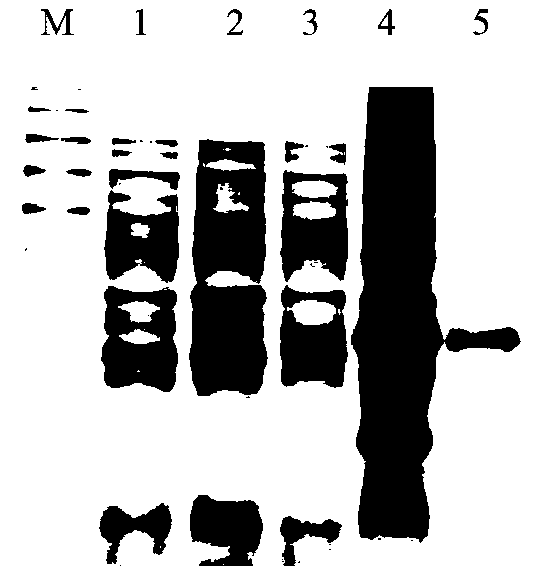
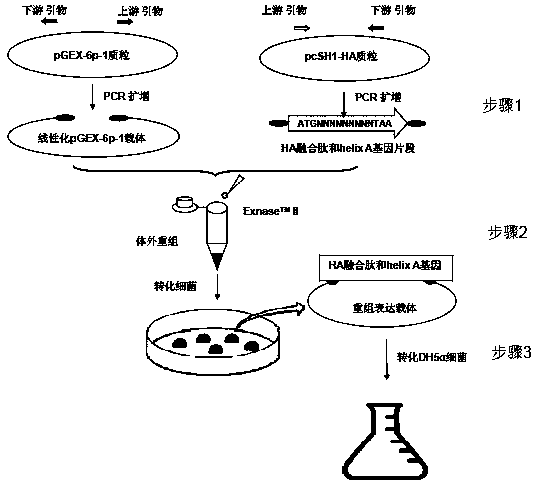
[0030]1. Design and amplify the primers containing the pGEX-6p-1 linearized vector, influenza virus HA fusion peptide and helix A gene fragment: the upstream primer for amplifying the pGEX-6p-1 linearized vector is located at positions 1022-1047 of the pGEX-6p-1 plasmid ; and with an extra TAA base at the 5' end; the downstream primer for amplifying the pGEX-6p-1 linearized vector is located at position 916-941 of the pGEX-6p-1 plasmid; and with an extra CAT base at the 5' end. Amplified influenza virus HA fusion peptide and helix A gene upstream primers added ATG GGA AGC as a strong promoter including 17 bases of HA fusion peptide and helix A gene, and 13 bases at the 5' end with pGEX-6p-1 Additional bases for the reverse complement of the downstream primers of the linearized vector; amplify the influenza virus HA fusion peptide and the helix A gene The downstream primers include the HA fusion peptide and the helix A gene stop codon TAA and its first 15 bases, and at the 5' en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com