Recombinant FCV antigen and feline calicirus genetic engineering subunit vaccine
An antigen and gene technology, applied in genetic engineering, antiviral agents, viruses, etc., can solve the problems of VLP and wild virus morphology, stability and immunogenicity differences, and achieve excellent immunogenicity, strong immunogenicity, and structure. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
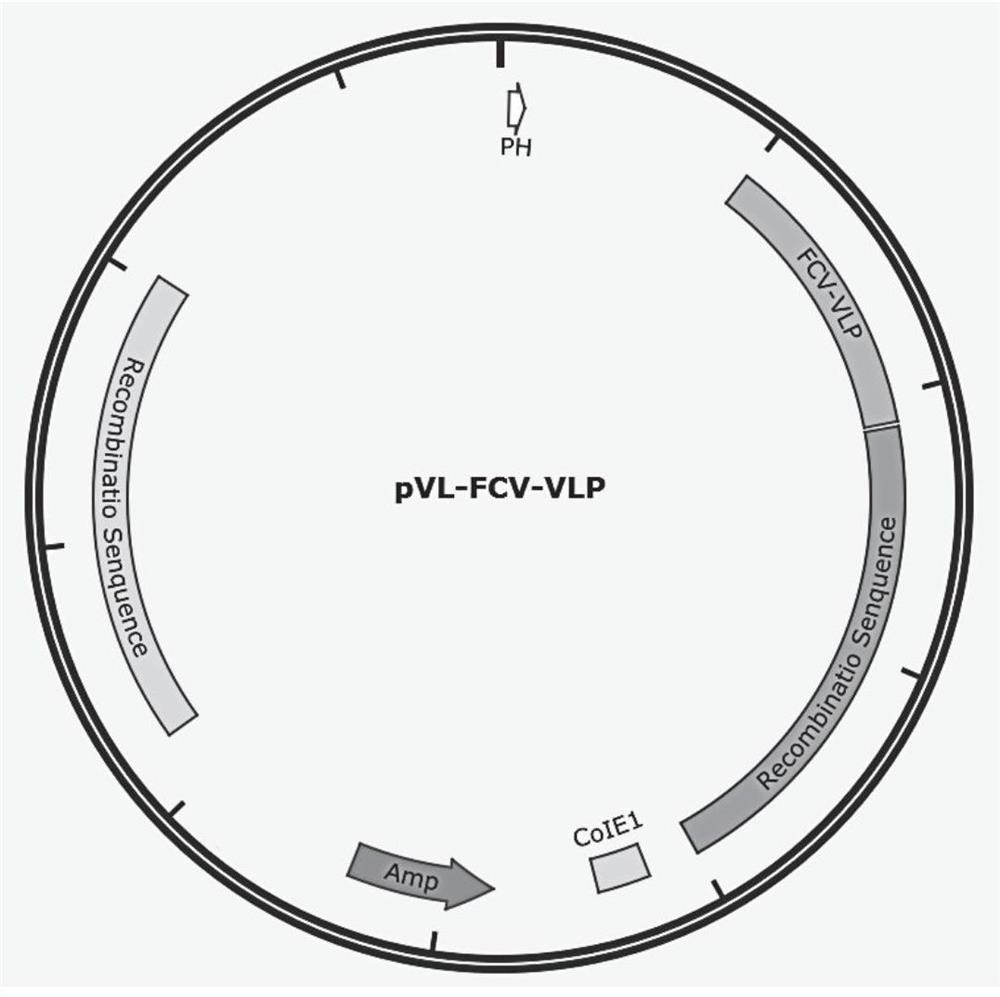
[0053] Example 1 Construction and Identification of Transfer Vector pVL-FCV-VLP
[0054] 1. FCV-VLP gene amplification and purification
[0055] The codon-optimized FCV-VLP gene (SEQ ID NO: 1) was synthesized in Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pMD19-T vector to obtain the pMD-FCV-VLP plasmid vector. With pMD-FCV-VLP plasmid as template, FCV-VLP-F, FCV-VLP-R carry out PCR amplification (the gene sequence of FCV-VLP-F, FCV-VLP-R as SEQ ID NO: 4 , 5), the amplification system is shown in Table 1.
[0056] Table 1 FCV-VLP gene amplification system
[0057]
[0058] The reaction conditions were: 94°C pre-denaturation for 5 minutes; 95°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
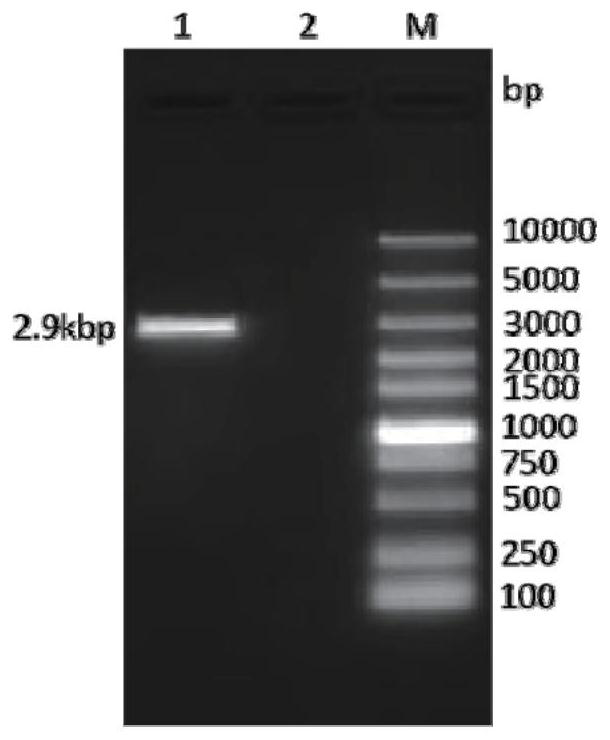
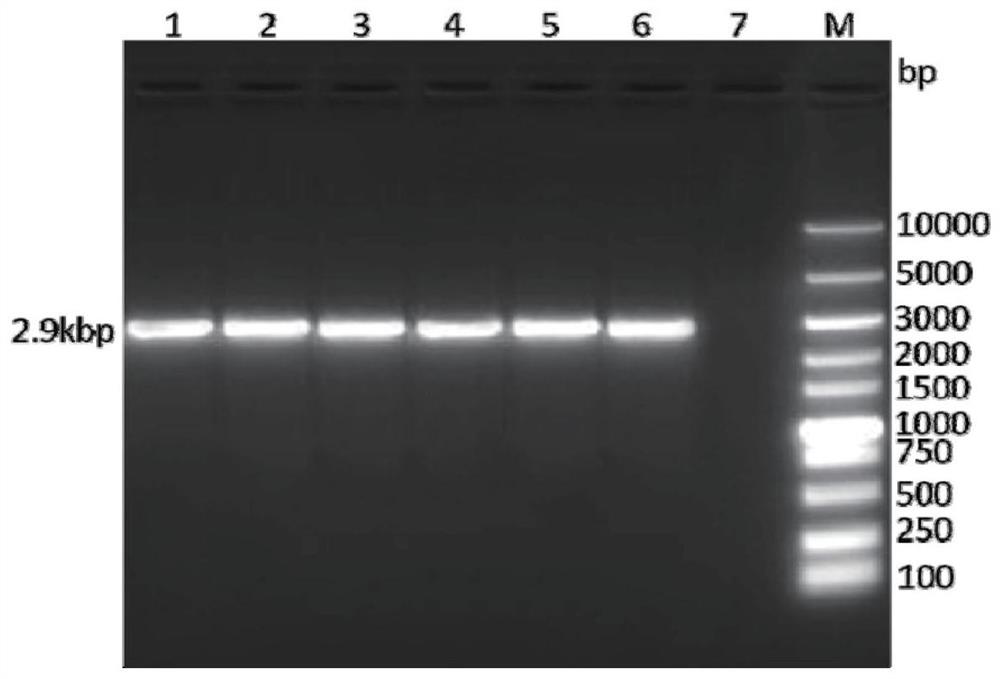
[0059] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as figure 1 As shown, the target band appeared at the position of 2.9kbp, the targe...
Embodiment 2
[0076] Embodiment 2 recombinant baculovirus rBac-FCV-VLP transfection and purification
[0077] 1. Transfection
[0078] Inoculate 2 × 10 in a tissue culture dish with a diameter of 6 cm 6 Sf9 cells, the cell confluence is 50-70%. Discard the medium after the cells adhere to the wall, and add 1.0ml of transfection buffer A. Mix 0.5 μg of BD BaculoGold LinearizedBaculovirus DNA and 2 μg of pVL-FCV-VLP plasmid in a sterile EP tube for 5 minutes, add 1.0ml of transfection reagent B, and mix well. Pipette the mixture dropwise into the tissue culture dish, incubate at 27°C for 4 hours, remove the transfection complex, wash the cell culture medium three times, add 3.0ml of fresh medium, seal the culture dish with parafilm, incubate at 27°C for 4 to 5 days and harvest Supernatant, that is, co-transfection virus supernatant.
[0079] 2. Plaque Purification
[0080] Inoculate 2.0 × 10 in a tissue culture dish with a diameter of 6 cm 6 ~2.5×10 6 Sf9 cells at room temperature for ...
Embodiment 3
[0086] Example 3 SDS-PAGE detection
[0087] The F0 generation recombinant baculovirus rBac-FCV-VLP cell culture harvested in Example 2 and the cell cultures of each control group were detected by SDS-PAGE, and Sf9 cells infected with empty baculovirus were used as a negative control. The specific operation is as follows: take 40 μl of harvested cell culture, add 10 μl of 5×loading buffer, bathe in boiling water for 5 minutes, centrifuge at 12000 r / min for 1 minute, take the supernatant and carry out SDS-PAGE gel (12% concentration gel) electrophoresis, After electrophoresis, the gel was stained and decolorized to observe the target band. Such as Figure 4 As shown, the rBac-FCV-VLP cell culture showed target bands at molecular weights of about 73kDa and 12kDa, and the protein expression levels of VP1 and VP2 showed a ratio of about 10:1, which was similar to wild virus; control group 1 had a molecular weight of about 73kDa The target band appeared at the target band; the co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com