Solid preparation and preparation method thereof
A technology for solid preparations and adhesives, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with non-active ingredients, etc., can solve problems such as the limitation of viscosity properties of active ingredients, shorten research and development time, improve quality, and improve industrialization. potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
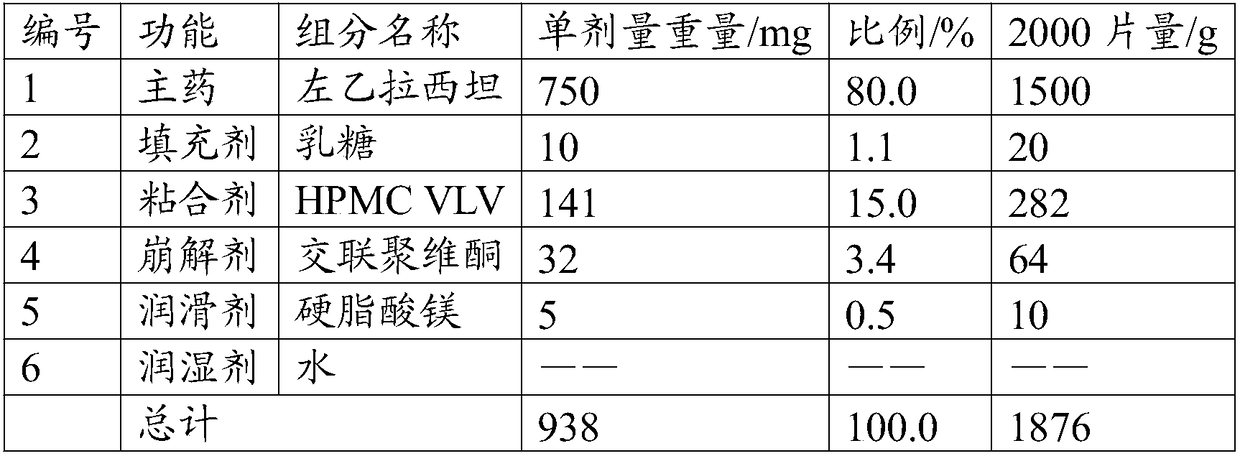
[0039] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0040]
[0041] Its preparation method is as follows:
[0042] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0043] (2) The binder is formulated into an aqueous solution with a mass concentration of 25%, added to the premixed material, granulated by a high-shear granulator, dried at 50°C for 10 minutes, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0044] (3) Add a lubricant to the dry intermediate and mix for 5 minutes to obtain granules.
[0045] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high drug loading.
[00...
Embodiment 2
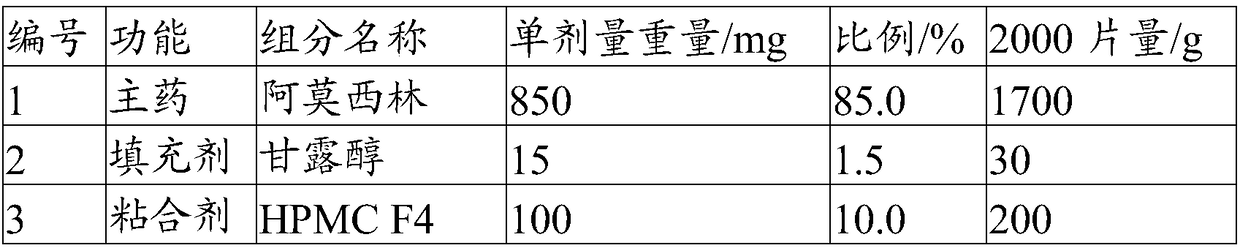
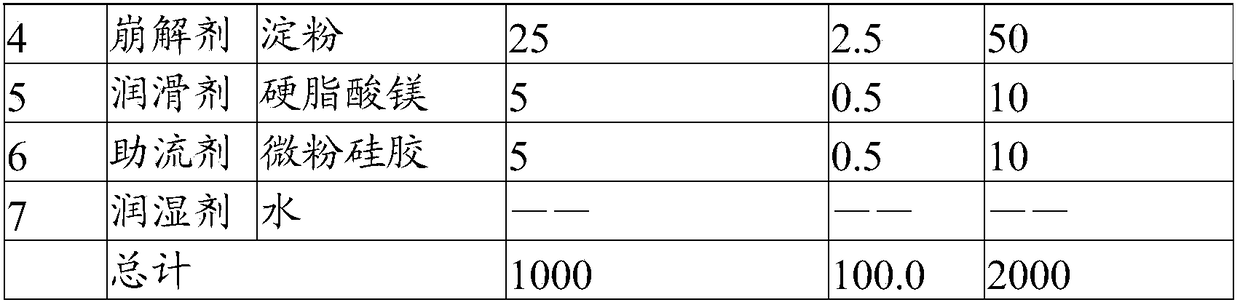
[0049] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0050]
[0051]
[0052] Its preparation method is as follows:
[0053] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0054] (2) The binder is formulated into an aqueous solution with a mass concentration of 20%, added to the premixed material, granulated by a high-shear granulator, dried at 50°C for 8min, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0055] (3) Add a lubricant and a glidant to the dry intermediate and mix for 5 minutes to obtain granules.
[0056] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high ...
Embodiment 3
[0060] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0061]
[0062] Its preparation method is as follows:
[0063] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0064] (2) The binder is formulated into an aqueous solution with a mass concentration of 20%, added to the premixed material, granulated by a high-shear granulator, dried at 55°C for 4min, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0065] (3) Add a lubricant and a glidant to the dry intermediate and mix for 5 minutes to obtain granules.
[0066] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high drug loading...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com