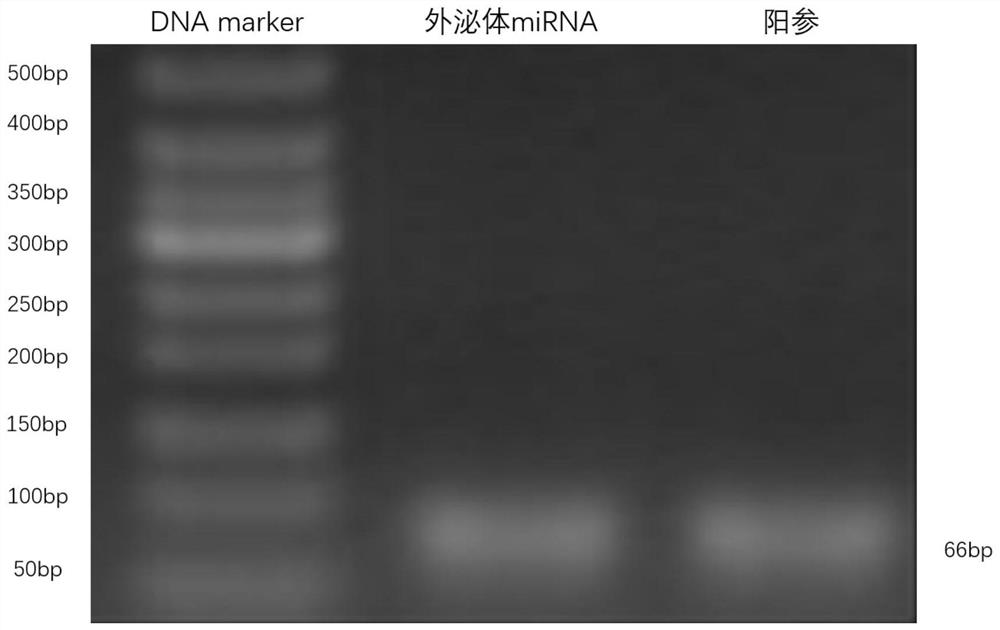
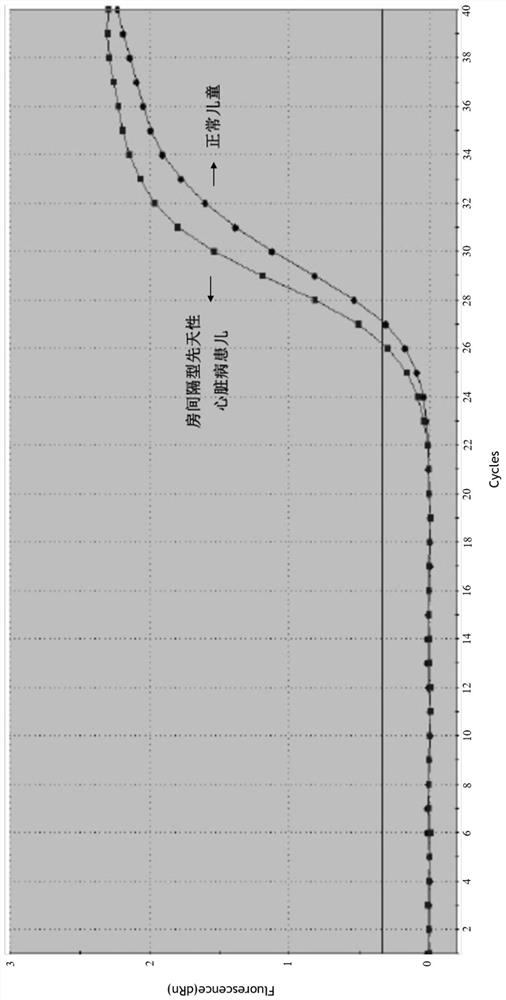
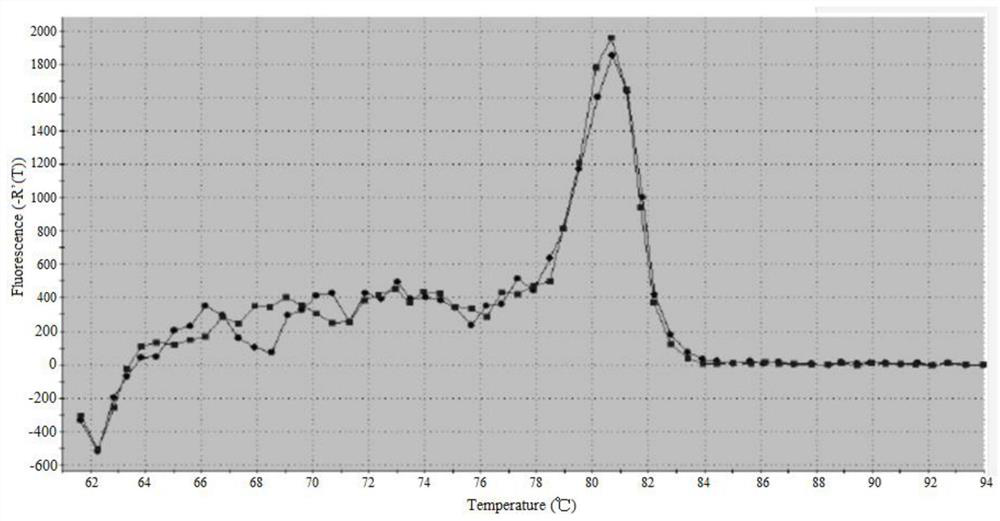
A serum/plasma exosomal miRNA marker associated with atrial septal congenital heart disease and its application
A congenital heart disease, exosome technology, applied in the field of biotechnology and diagnosis, can solve the problem of atrial septal congenital heart disease specific miRNA research that cannot be used as a marker for specific diagnosis of atrial septal congenital heart disease Few, can not be used as a wide range of screening and other issues, to achieve the effect of high reverse transcription efficiency, high sensitivity, strong accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Experimental materials and methods
[0042] 1.1 Case selection
[0043] 1.1.1 Diagnostic criteria
[0044] Diagnosis of atrial septal congenital heart disease Children with simple atrial septal congenital heart disease diagnosed by echocardiography in the hospital.
[0045] 1.2 Case information
[0046] From December 2015 to December 2017, a total of 30 patients with atrial septal congenital heart disease who met the inclusion criteria were collected in this study. All cases came from the Department of Cardiothoracic Surgery of Nanjing Children's Hospital and were confirmed by pathological examination. The age of the patients ranged from 1 to 13 years, with a median age of 2.5 years. The normal control group ("normal" in this study refers to non-cardiac developmental malformations) 29 cases, aged 1-11 years, with a median age of 4 years, were healthy volunteers from the Department of Pediatrics, Nanjing Children's Hospital Affiliated to Nanjing Medical University....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com