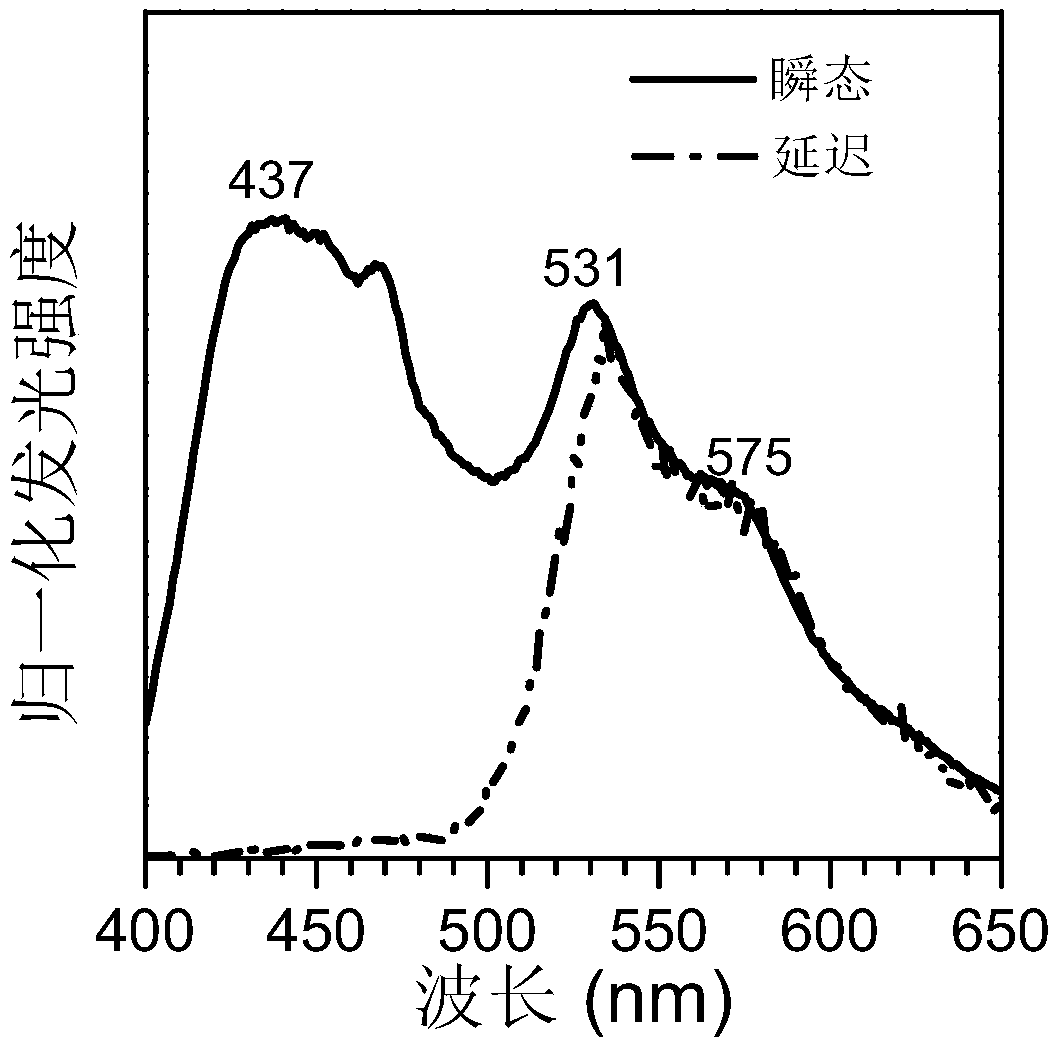
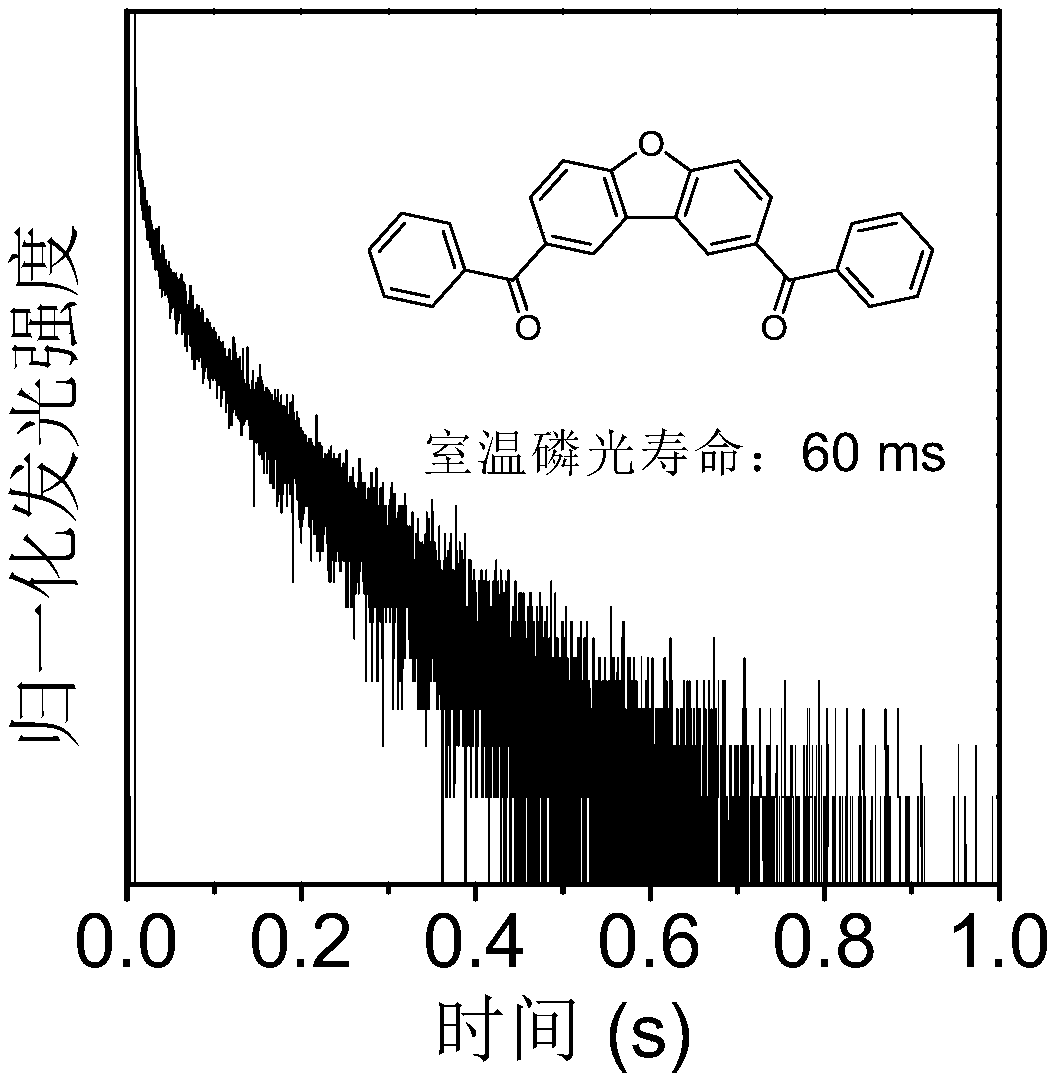
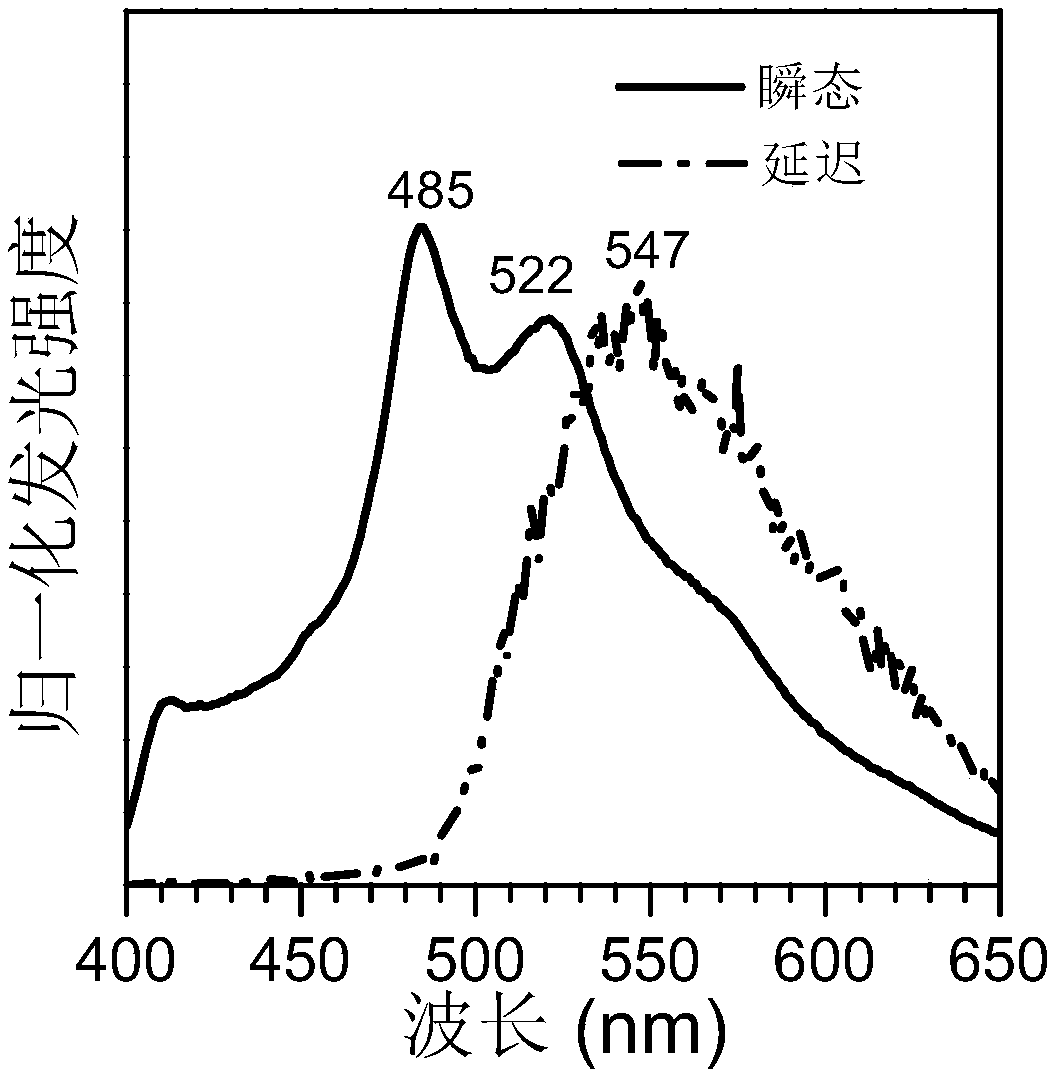
Carbonyl-containing room temperature phosphorescent material based on dibenzofuran as well as preparation method and application thereof
A technology of dibenzofuran and room temperature phosphorescence, which is applied in the direction of luminescent materials, chemical instruments and methods, and sustainable buildings. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 The preparation and synthesis route of carbonyl-containing room temperature phosphorescent material DBF-2,8-DBPM based on dibenzofuran is as follows:
[0048]
[0049] Add dibenzofuran (0.84g, 5.0mmol) and sodium chloride (0.87g, 15.0mmol) solid powder into the flask, continue to add aluminum trichloride (2.00g, 15.0mmol), and stir evenly with a magnet, Add benzoyl chloride (1.44mL, 12.5mmol), heat to 150°C, and react at this temperature for 4 hours. After the reaction, extract three times with dichloromethane and water, take the organic phase to concentrate, add anhydrous sodium sulfate to remove water, adsorb on silica gel powder, and separate by column chromatography to obtain 1.70 g of the product with a yield of 90%.
[0050] Dissolve the product in dichloromethane, add an equal volume of n-hexane, wait for the solution to volatilize slowly, precipitate crystals, and filter to obtain crystals.
[0051] DBF-2,8-DBPM: 1 H NMR (500MHz, Chloroform-d) δ8....
Embodiment 2
[0053] Example 2: Preparation and separation of carbonyl-containing room temperature phosphorescent materials DBF-2,8-DB(4-Cl)PM and DBF-2,7-DB(4-Cl)PM based on dibenzofuran
[0054] The synthetic route is as follows:
[0055]
[0056] Add dibenzofuran (1.68g, 10.0mmol) and sodium chloride (1.75g, 30.0mmol) solid powder into the flask, continue to add aluminum trichloride (4.00g, 30.0mmol), and stir evenly with a magnet, Add p-chlorobenzoyl chloride (2.55mL, 20mmol), heat to 150°C, and react at this temperature for 4 hours. After the reaction, extract three times with dichloromethane and water, take the organic phase and concentrate, add anhydrous sodium sulfate to remove moisture, adsorb on silica gel powder, and separate by column chromatography to obtain 3.70 g of the mixed product of the two, with a total yield of 83%.
[0057] Dissolve the mixed product in 200mL of dichloromethane, heat to dissolve it completely, add 200mL of n-hexane, let it stand still to volatiliz...
Embodiment 3
[0059] Example 3: Preparation and separation of carbonyl-containing room temperature phosphorescent materials DBF-2,8-DB(4-Br)PM and DBF-2,7-DB(4-Br)PM based on dibenzofuran
[0060] The synthetic route is as follows:
[0061]
[0062] Add dibenzofuran (1.68g, 10.0mmol), sodium chloride (1.75g, 30.0mmol) and aluminum trichloride (4.00g, 30.0mmol) solid powder into the flask, stir evenly with a magnet, then add the Bromobenzoyl chloride (4.39g, 20.0mmol), heated to 150°C, reacted at this temperature for 4 hours. After the reaction, extract three times with dichloromethane and water, take the organic phase and concentrate, add anhydrous sodium sulfate to remove moisture, adsorb on silica gel powder, and separate by column chromatography to obtain 3.90 g of the mixed product of the two, with a total yield of 70%.
[0063] Dissolve the mixed product in 200mL of dichloromethane, heat and ultrasonically dissolve it completely, add 200mL of n-hexane, let it stand to volatilize t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com