Stable high-drug-loading-ratio lidocaine transdermal patch and preparation method thereof
A technology of lidocaine and transdermal patches, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as crystal transformation or growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A lidocaine-containing transdermal patch comprises a polymer matrix layer; the polymer matrix layer contains active ingredient lidocaine and acrylic pressure-sensitive adhesive without functional groups. Further, the patch also includes a backing layer and a protective layer; the polymer matrix layer is located between the backing layer and the protective layer.
[0048] After mixing the lidocaine and the acrylic pressure-sensitive adhesive without functional groups, the temperature is raised to about 60-80° C., so that the two form a homogeneous blend dispersed with each other to form the polymer matrix layer.
[0049] In the present embodiment, the weight contents of lidocaine and acrylic pressure-sensitive adhesive without functional groups in the polymer matrix layer are 60% and 39.8% respectively; 0.2%) antioxidants (such as BHA).
[0050] In this embodiment, the lidocaine is lidocaine free base; the acrylic pressure-sensitive adhesive without functional groups is...
experiment example 1
[0059] Experimental example 1 Stability experiment
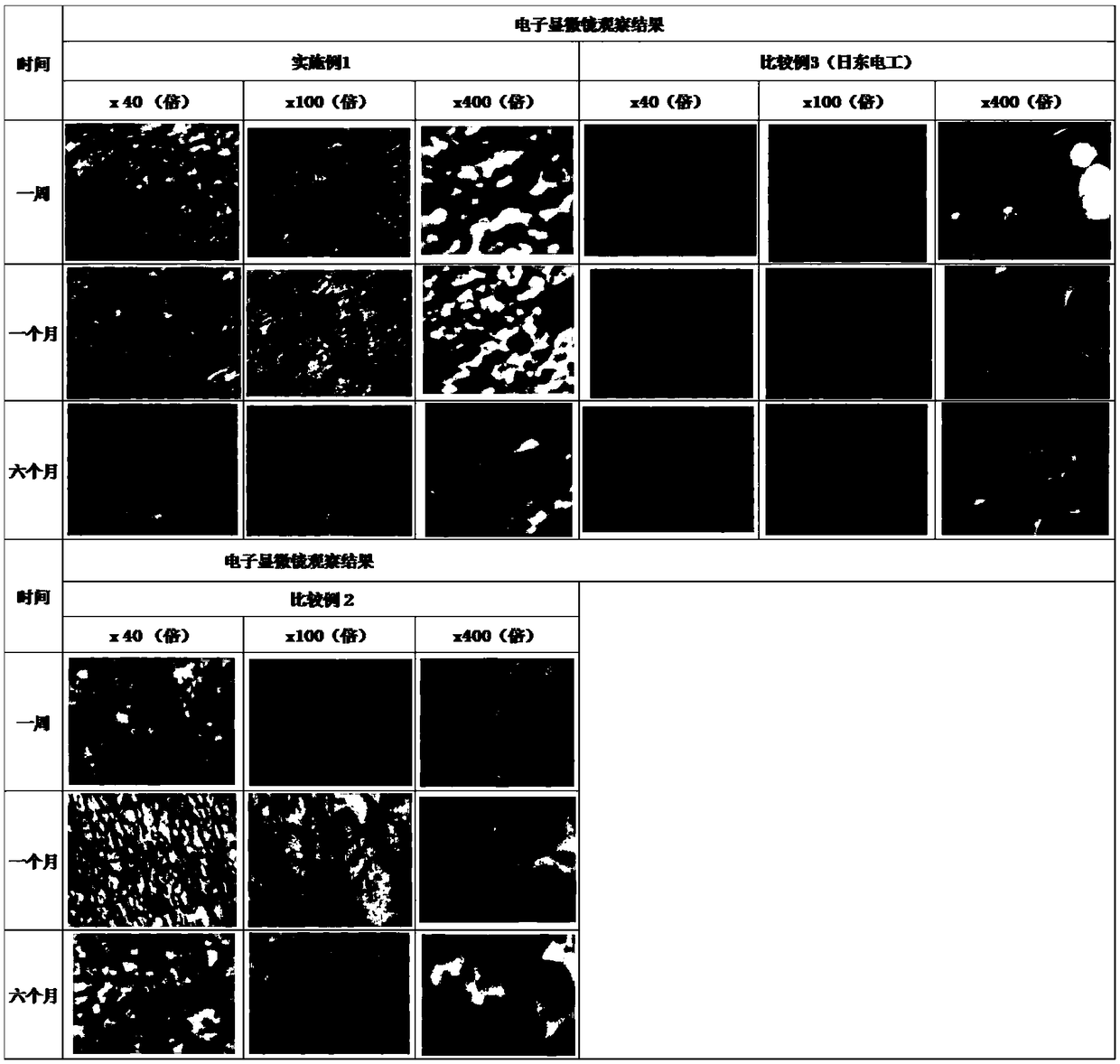
[0060] The patches of Example 1, Comparative Example 2, and Comparative Example 3 were placed under the same storage conditions (30 ± 2° C., 60% ± 10% RH), and were regularly observed by an electron microscope. The observation results are shown in figure 1 .
[0061] The results of continuous observation showed that the active ingredients of the patches prepared in Example 1 and Comparative Example 2 of the present invention did not change significantly during the observation period, and lidocaine was uniformly dispersed in the polymer matrix in the form of tiny particles, and different multiples of optical No lidocaine particles (crystals) exist under the microscope, and no crystallization phenomenon is observed, indicating that the patch of the present invention has good stability. In the lidocaine patch of comparative example 3, crystal particles of lidocaine can be clearly observed, especially under high magnification o...
experiment example 2
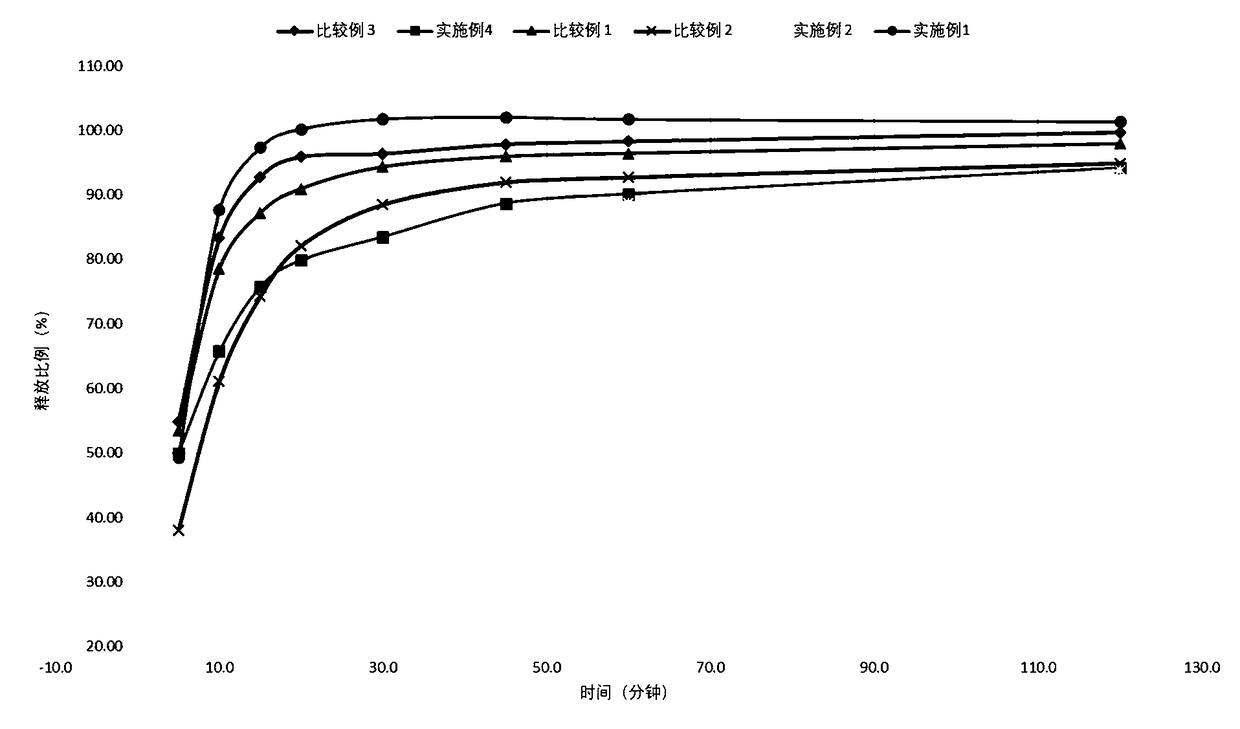
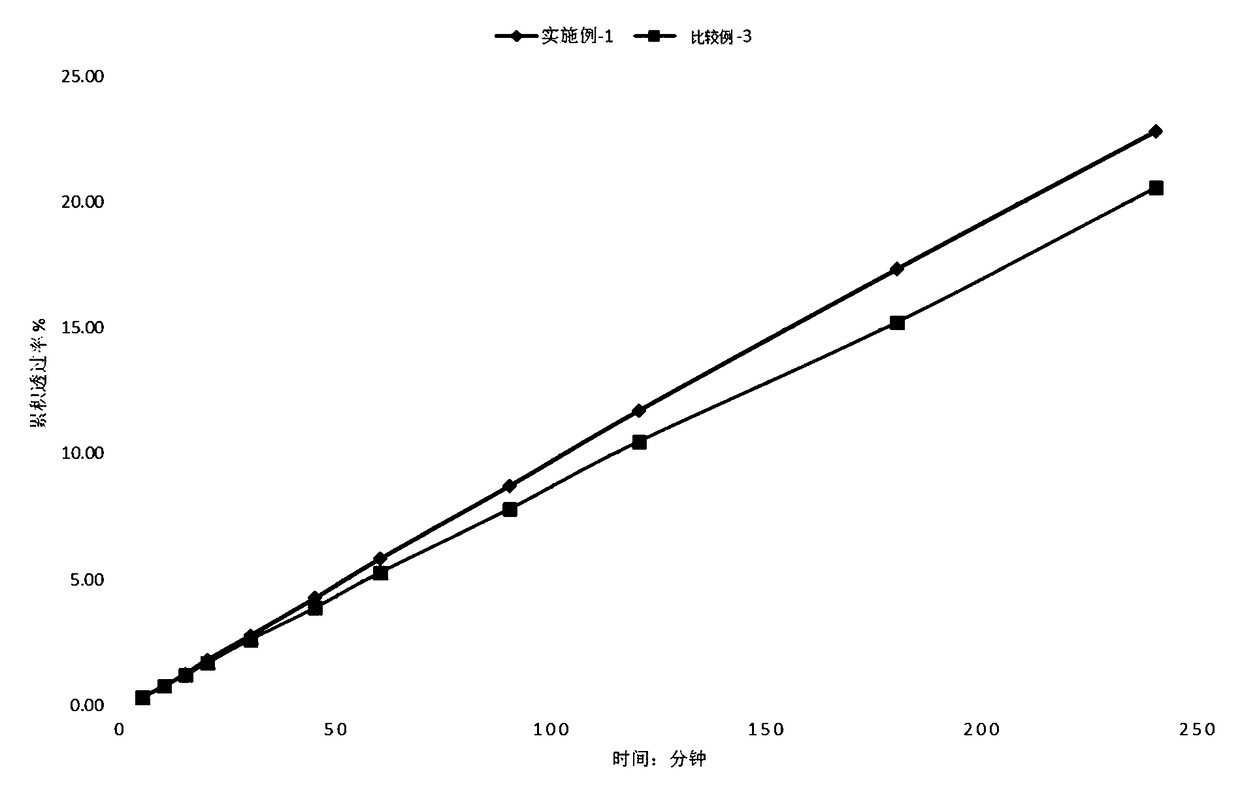
[0062] Experimental example 2 in vitro release test
[0063] In vitro release is an essential performance indicator of a patch, reflecting the interaction of the active ingredient with other components in the polymer matrix. The overall properties of the polymer matrix, the interactions between lidocaine and polymer PSA, and other components such as hydrogen bonds, ion pairs, van der Waals forces, etc., lead to different flow behaviors of lidocaine in the polymer matrix. In vitro release is the basis of transdermal absorption, and only appropriate release capacity can meet specific transdermal absorption requirements.
[0064] According to the dissolution and release determination method (Chinese Pharmacopoeia 2015 edition fourth general rule 0931 fourth method - paddle-disc method), with PBS as the dissolution medium, the medium temperature is 32 ℃, 25 rpm, operated according to law, after 20 In 20 minutes, take 5ml of the solution, filter, and get the continued filtrate to ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap