The application of the marker clptm1 in the diagnosis and treatment of epilepsy
A technology in epilepsy and samples, which is applied in the field of CLPTM1 gene mutation and its application, and can solve problems such as inability to diagnose before onset
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1. Research population
[0070] In order to study the relationship between the CLPTM1 gene and epilepsy patients, two groups were set up for analysis, namely the patient group and the control group, as follows:
[0071] 1.1 Patient Group
[0072] The patient group selected children with epilepsy from Peking University First Hospital as the research object, and the specific inclusion criteria are as follows:
[0073] 1) No clear history of perinatal brain injury;
[0074] 2) No clear history of cerebral hypoxia, ischemia, central nervous system infection or head trauma;
[0075] 3) Exclude known genetic metabolic diseases and genetic neurodegenerative diseases;
[0076] 4) Normal karyotype;
[0077] 5) The known epilepsy gene test is negative.
[0078] Collect the clinical data of the enrolled children and sign an informed consent form with the guardians of the children. At the same time, the peripheral blood of the children and their parents is collected, and the g...
Embodiment 2
[0094] Example 2 sequence verification
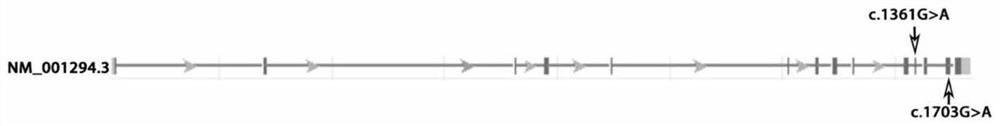
[0095] The method of Sanger sequencing was used to verify and detect the two mutation sites of the CLPTM1 gene, and the verification results were as follows Figure 4 and Figure 5 shown, according to Figure 4 , 5 The verification results shown show that exon missense mutations c.1703G>A and c.1361G>A occurred in the CLPTM1 gene of patients with epilepsy.
Embodiment 3
[0096] Example 3 Cytology Experiment Verification
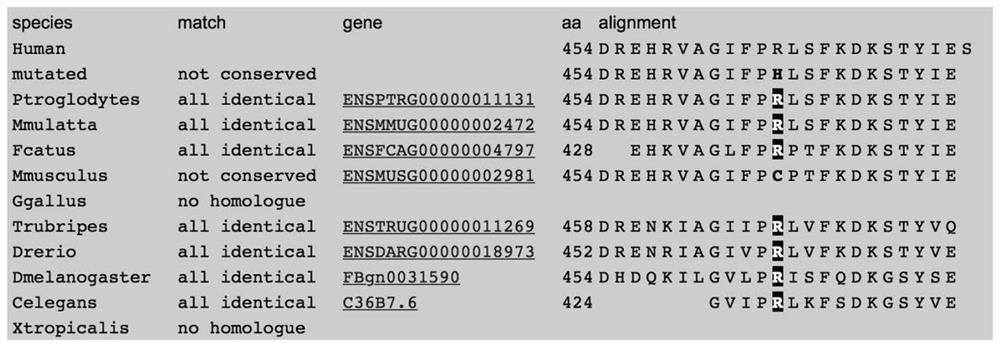
[0097] GABAergic neurons are the main inhibitory neurons in the central nervous system and are closely related to epilepsy. The decline of GABAergic neuron function will lead to excessive discharge of neuron network, thereby causing epilepsy. Therefore, the present invention uses cytology experimental method to verify the influence of p.R568Q and p.R454H mutation on GABA current, so as to judge whether the mutation is related to epilepsy. Relationship.
[0098] In order to explore the effects of the two single amino acid substitutions caused by the above two mutations (p.R568Q and p.R454H mutations) on GABAergic neurons, this example constructed wild-type (WT) and two kinds of R568Q-expressing Or the mutant plasmid of the R454H mutation site (c.1703G>A: p.R568Q and c.1361G>A: p.R454H), and transiently transfect CRL-2029 cells with the constructed plasmid. After the successful transfer of the constructed plasmid into CRL-202...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com