Novel FBN2 gene mutation marker and application thereof in CCA auxiliary diagnosis
An auxiliary diagnosis and gene technology, applied in the field of molecular biology, can solve the problem of not meeting the diagnostic criteria of CCA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
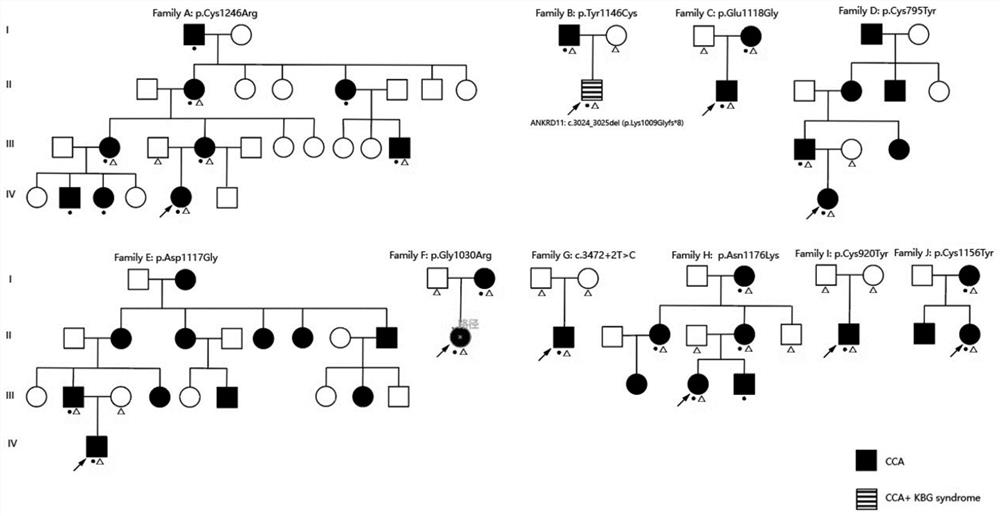
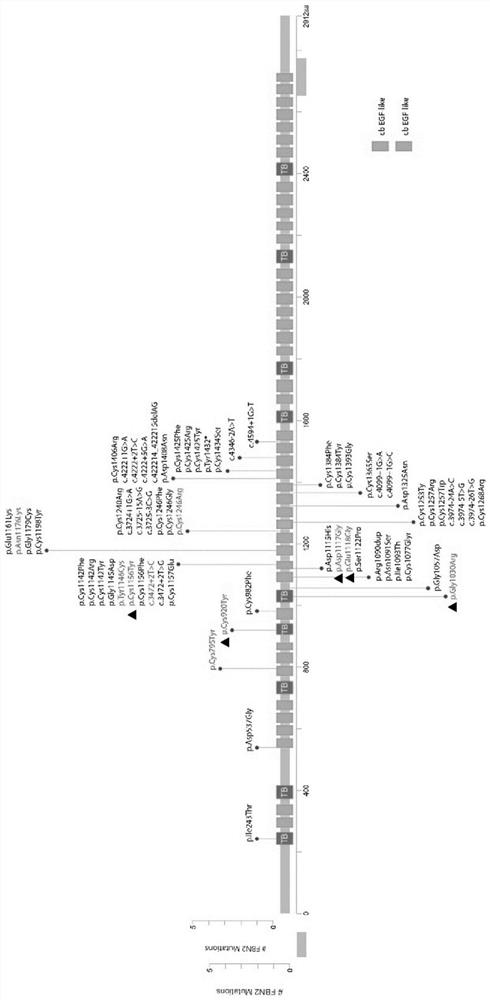
[0072] Example 1 Screening of Gene mutations associated with CCA FBN2 embodiment
[0073] 1, study
[0074] Recruited 10 clinical and molecular diagnosis of CCA probands were from Beijing Jishuitan Hospital, Beijing Union Medical College Hospital Hand Surgery and Orthopedics were assessed by two reviewers (LS and YH) by physical examination or X-ray photographs proband those clinical phenotypes, and their families a related investigation, all subjects or their guardians signed an informed consent form, the present study was Beijing Ji Shui Tan hospital review Board approval.
[0075] 2, DNA extraction
[0076] After informed consent, patients and their family members drawn peripheral blood system, dipotassium ethylenediaminetetraacetic acid (EDTA-K2) anticoagulant, according to manufacturer's instructions, using the DNeasy blood / tissue genomic DNA extraction kit (Germany , QIAGEN) to extract DNA, A 260 / A 280 Ratio in the range between 1.8 to 2.0.
[0077] 3, genetic testing
...
Embodiment 2
[0090] Example embodiment were verified by sequencing 2Sanger
[0091] 1, primer design
[0092] Primer 5 employed for PCR primer design software. The primer sequences used are as follows:
[0093] c.3353A> G, c.3350A> G, c.3467G> A with primers
[0094] FBN2-1-F: GTATGGTTTCAAGCTGGCGA (SEQ ID NO: 1)
[0095] FBN2-1-R: TTTTGGCAGTTGGGGAAAGG (SEQ ID NO: 2)
[0096] c.2759G> A with primers
[0097] FBN2-2-F: AGAAGGCCTGCTTATGTACCA (SEQ ID NO: 3)
[0098] FBN2-2-F: GGTAAGAGCTCCCCAACCTT (SEQ ID NO: 4)
[0099] c.3088G> A with primers
[0100] FBN2-3-F: ACCTTTGTAAAATGGCCGCC (SEQ ID NO: 5)
[0101] FBN2-3-R: ACGCTTGACATGTGACTGT (SEQ ID NO: 6)
[0102] 2, Sanger sequencing
[0103] The experimental steps are as follows:
[0104] a) 3 arranged PCR System according to the table;
[0105] Table 3 Gene PCR System
[0106]
[0107] b) PCR reaction conditions shown in Table 4;
[0108] Table 4 FBN2 gene PCR reaction conditions
[0109]
[0110] c) arranged in a 1% agarose gel;
[0111] d) Aft...
Embodiment 3
[0116] Example 3 verification of causative mutations FBN2 CCA by clinical scoring system
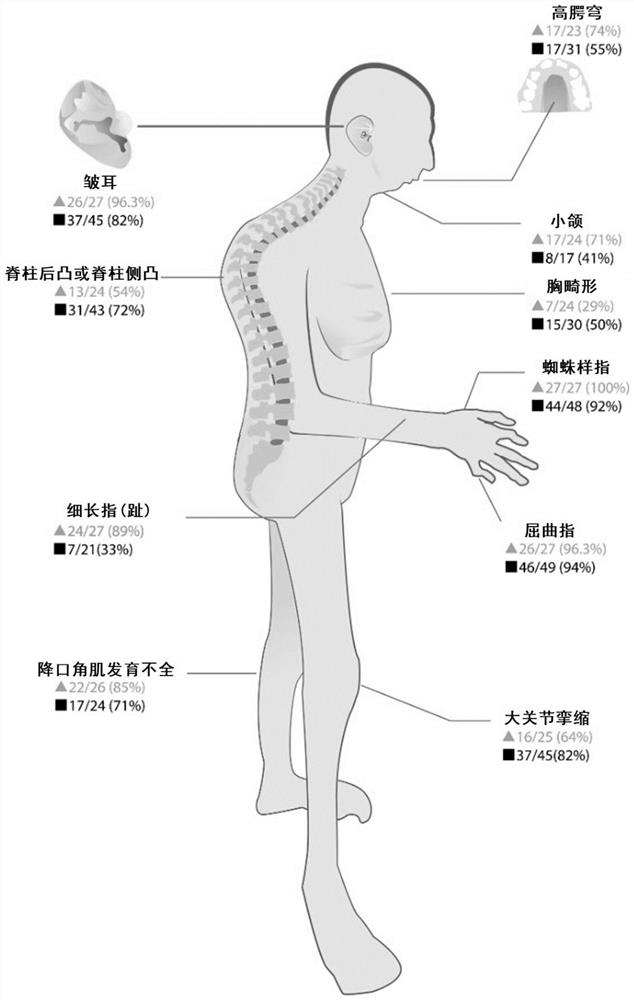
[0117] In the present embodiment, in accordance with the subject phenotypic records, is calculated for each subject CCA clinical score, based on the results of the clinical score, pathogenic FBN2 gene according to the present invention was verified, the clinical CCA the ratings Document "Meerschaut I, De Coninck S, Steyaert W, et al.A clinical scoring system forcongenital contractural arachnodactyly [J] .Genetics in Medicine, 2020,22 (1): 124-131." the clinical score reported CCA the system computing.
[0118] 1, experimental method
[0119] Specific scoring criteria in Table 5, score ≥7 points highly suspicious CCA.
[0120] Table 5 CCA clinical score
[0121]
[0122]
[0123] 2, experimental results
[0124] 11 failure patients clinical phenotype evaluation excluded, the remaining 16 patients with clinical phenotype CCA score calculation see Figure 9 , Up to 17 points, 8 points the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com