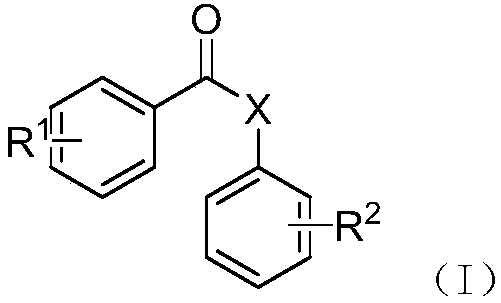
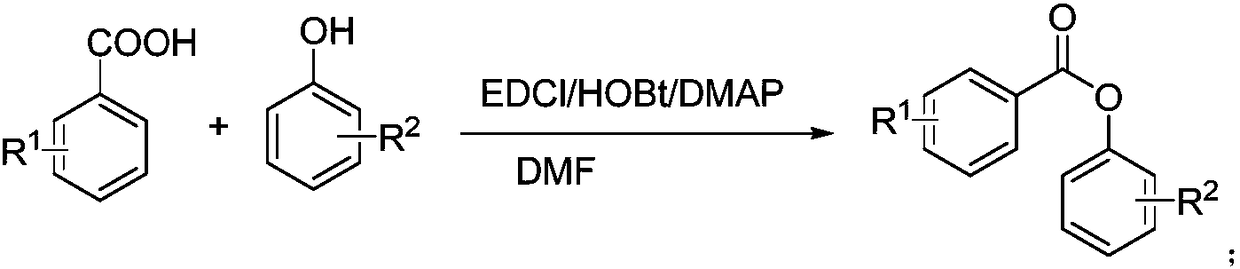
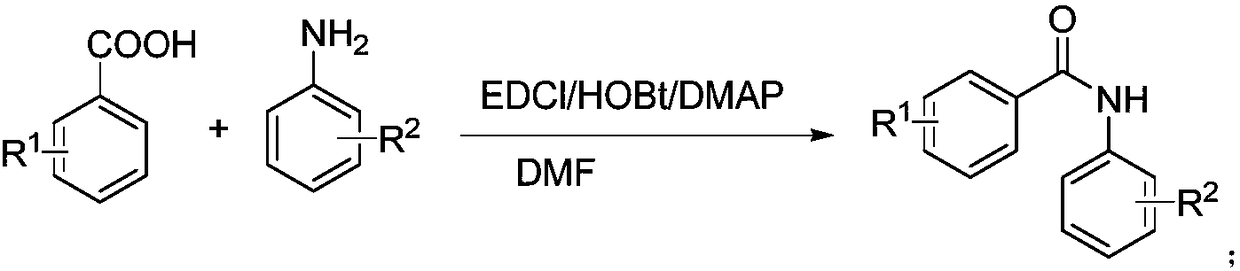
Benzoic acid derivatives and application thereof
A derivative, benzoic acid technology, applied in the field of benzoic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (preparation CJ-1-1)
[0034] The structural formula is (CJ-1-1) The preparation method of compound is as follows:
[0035]
[0036] Dissolve 1mmol of 3-nitro-4-methoxybenzoic acid in DMF, add 1.2mmol of TEA, then add 1.1mmol of HOBT, 1.2mmol of EDCI, and finally add 1mmol of 3,4,5-trimethoxyaniline. TLC monitoring, after the reaction was completed, water was added to precipitate a yellow solid. The obtained product was identified by nuclear magnetic resonance and mass spectrometry, and the identification result was 1 H NMR (CDCl 3 )δ7.66(s,1H),δ7.275-7.281(d,1H),δ7.229-7.256(m,1H),δ6.97(s,2H),δ6.85-6.87(d,1H ),δ3.95(s,3H),δ3.91(s,6H),δ3.86(s,3H).MS(ESI)m / z 361.57(M-H) - .Anal.(C 17 h 14 N 2 o 3 ) C, H, N, O. According to the above identification results, the obtained product is CJ-1-1.
Embodiment 2
[0037] Embodiment 2 (preparation CJ-1-2)
[0038] The structural formula is (CJ-1-2) The preparation method of the compound is the same as CJ-1-1, and the obtained product is identified by nuclear magnetic resonance and mass spectrometry, and the identification result is 1 H NMR(DMSO)δ8.10-8.11(dd,1H),δ7.98-8.01(dd,1H),δ7.84(s,1H),δ7.15(dd,1H),δ7.10(s ,2H),δ4.00(s,3H),δ3.96(s,6H),δ3.94(s,3H).MS(ESI) m / z 362.47(M+H) + .Anal.(C 17 h 18 N 2 o 7 ) C, H, N, O. According to the above identification results, the obtained product is CJ-1-2.
Embodiment 3
[0039] Embodiment 3 (preparation CJ-1-3)
[0040] The structural formula is (CJ-1-3) The preparation method of compound is as follows:
[0041]
[0042] Dissolve 1mmol 3-nitro-4-methoxybenzoic acid in DMF, add TEA1.2mmol, add HOBT1.1mmol, EDCI1.2mmol, DMSP 0.02mmol, and finally add 3,4,5-trimethoxyphenol 1 mmol. TLC monitoring, after the completion of the reaction, add water to precipitate a solid. The obtained product was identified by nuclear magnetic resonance and mass spectrometry, and the identification result was 1 H NMR (CDCl 3 )δ8.69(s,1H),δ8.37-8.39(d,1H),δ7.22-7.24(d,1H),δ6.49(s,2H),δ4.10(s,3H), δ3.88(s,9H).MS(ESI)m / z 385.70(M+Na) +.Anal.(C 17 h 17 NO 8 ) C, H, N, O. According to the above identification results, the obtained product is CJ-1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com