Preparation method and electrocatalytic application of nickel bimetallic nanometer material
A bimetallic nano-electrocatalysis technology, applied in the field of electrochemistry, can solve the problems of inability to popularize industrial applications, complicated reaction conditions, etc., and achieve the effects of excellent electrochemical properties and chemical catalytic properties, cheap and easily available raw materials, and simple synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 1.4540g Ni(NO 3 ) 2 ·6H 2 O and 0.5097g AgNO 3 Dissolve in a mixed solution of 10mL deionized water and 20mL absolute ethanol and keep stirring, then slowly add dropwise a mixed solution of 30mL NaOH with a concentration of 1mol / L and 20mL with a concentration of 50vol% hydrazine hydrate, and react at 60°C for 3h , and then ultrasonically wash the product with deionized water and absolute ethanol, respectively, until the supernatant is clear and transparent, and finally vacuum-dry at 60°C to obtain a Ni / Ag 5 / 3 bimetallic nanomaterial.
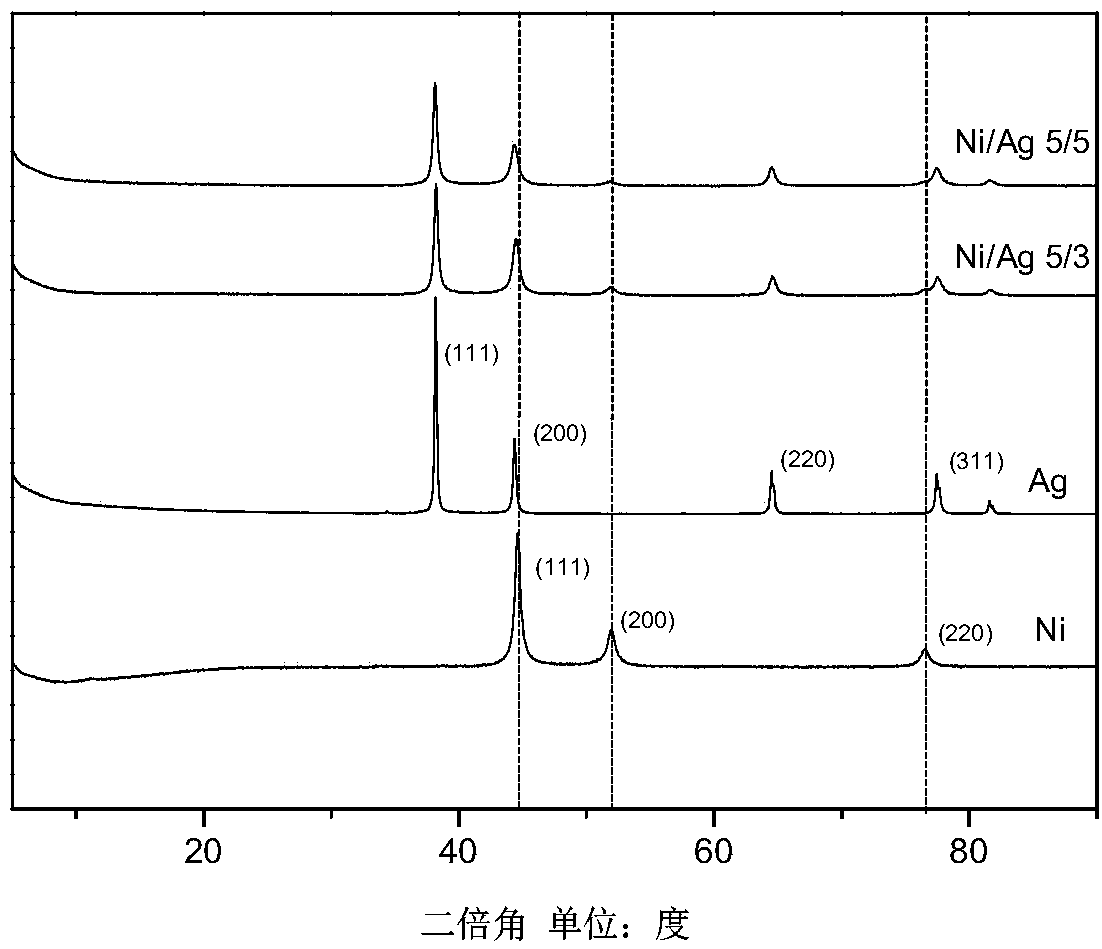
[0025] See attached figure 1 , the above products are characterized by XRD curves, Ni / Ag 5 / 3 bimetallic nanomaterials have (111), (200) and (220) characteristic peaks of nickel and silver (111), (200), (220) and The characteristic peak of (311) shows that the product of embodiment 1 is the pure metal nanomaterial of nickel and silver.
[0026] See attached figure 2 , the above product was characterized by a scanning electron ...
Embodiment 2
[0028] Take 0.1g of the above-prepared Ni / Ag 5 / 3 bimetallic nanomaterial and add 0.2mL sodium carboxymethylcellulose sol, ultrasonically disperse and evenly coat both sides of 2cm×2.2cm carbon paper, and dry naturally to obtain Ni / Ag 5 / 3 solid electrodes made of bimetallic nanomaterials.
[0029] Add 20 mL of anhydrous N,N-dimethylformamide (DMF), 1 mmol of acetophenone, 2 mmol of tetra-n-butylammonium iodide (TBAI), 0.05 mmol of cinchonidine ( CD), 0.05mmol n-butanol, continuous and slow introduction of CO 2 and stir. Then insert a solid electrode made of Ni / Ag 5 / 3 bimetallic nanomaterials as the cathode, and a polished magnesium rod as the anode, and connect it to a galvanostat to form a circuit path, select a constant direct current of 10mA, and a current density of 2.5mA / cm 2 , until the end of the electrocarboxylation reaction. After the solvent was removed by rotary evaporation, 10 mL of 1 mol / L HCl was added to acidify, extracted three times with 30 mL of anhydrou...
Embodiment 3
[0031] Add 20 mL of anhydrous N,N-dimethylformamide (DMF), 1 mmol of acetophenone, 2 mmol of tetra-n-butylammonium iodide (TBAI), 0.05 mmol of L-(+) - Tartaric acid (L-(+)-TA) and 0.05mmol n-butanol, continuous slow passage of CO 2 and stir. Then insert a solid electrode made of Ni / Ag 5 / 3 bimetallic nanomaterials as the cathode, and a polished magnesium rod as the anode, and connect it to a galvanostat to form a circuit path, select a constant direct current of 10mA, and a current density of 2.5 mA / cm 2 , until the end of the electrocarboxylation reaction. After removing the solvent by rotary evaporation, add 10 mL of 1 mol / L HCl to acidify, and extract with 30 mL of anhydrous ether three times, then wash once with 10 mL of saturated NaCl solution, add anhydrous magnesium sulfate to dry for 1 hour, and then remove the ether by rotary evaporation to obtain the target Product 2-hydroxyl-2-phenylpropionic acid (2-hydroxy-2-phenylpropionic acid), its productive rate is 17%, ee ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com