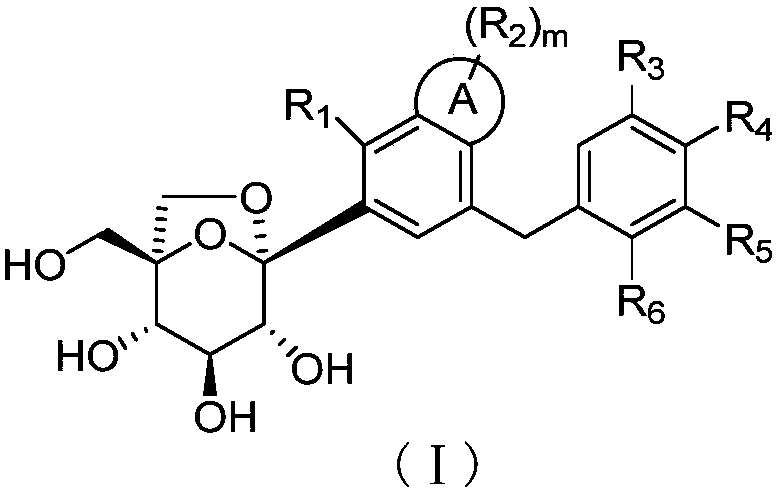
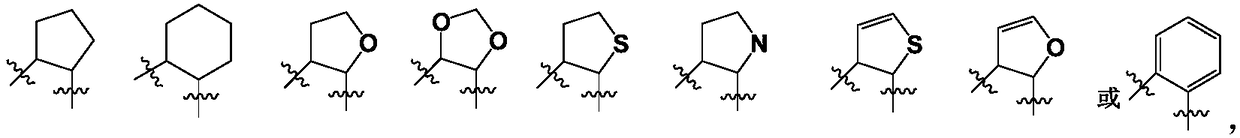
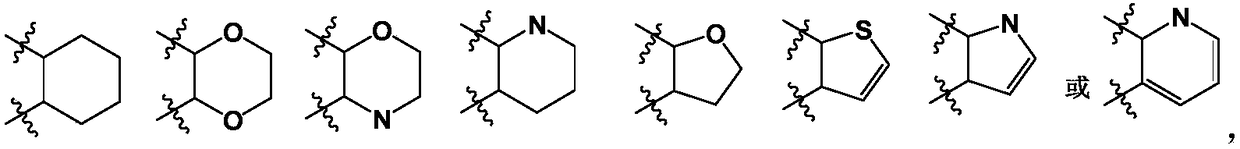
SGLTs (sodium-dependent glucose transporters 2) inhibitor, preparation method thereof and pharmaceutical application of inhibitor
A technology of pharmacy, compound, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 (1S, 2S, 3S, 4R, 5S)-1-(hydroxymethyl)-5-(7-(4-methoxybenzyl)-4-methyl-2,3-dihydro-1H Preparation of -inden-5-yl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol
[0111]
[0112] Step 1: Preparation of (6-bromo-7-methyl-2,3-dihydro-1H-inden-4-yl)(4-methoxyphenyl)methanone
[0113]
[0114] N 2 Under protection, 6-bromo-7-methyl-2,3-dihydro-1H-indene-4-carboxylic acid (6.9 g, 27.1 mmol) was dissolved in DCM (30 mL), the solution was cooled to 0 °C, then Will (COCl) 2 (3.5 mL, 41.4 mmol) in DMF (0.10 mL) was slowly added dropwise. The reaction solution was stirred overnight at room temperature. After the reaction solution was concentrated, the oil pump was pumped dry for half an hour. The resulting product was dissolved in DCM (50 mL), then cooled to 0 °C, anisole (3.5 g, 32.4 mmol) and AlCl were added 3 (4.5 g, 33.8 mmol). After the reaction solution was warmed to room temperature, it was stirred at room temperature for 4 hours. The reaction solution w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com