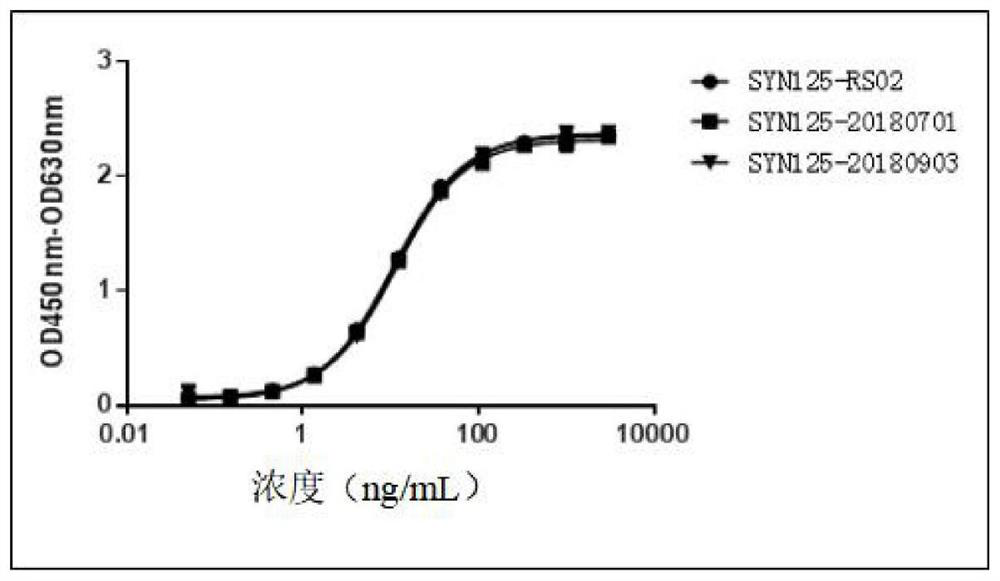
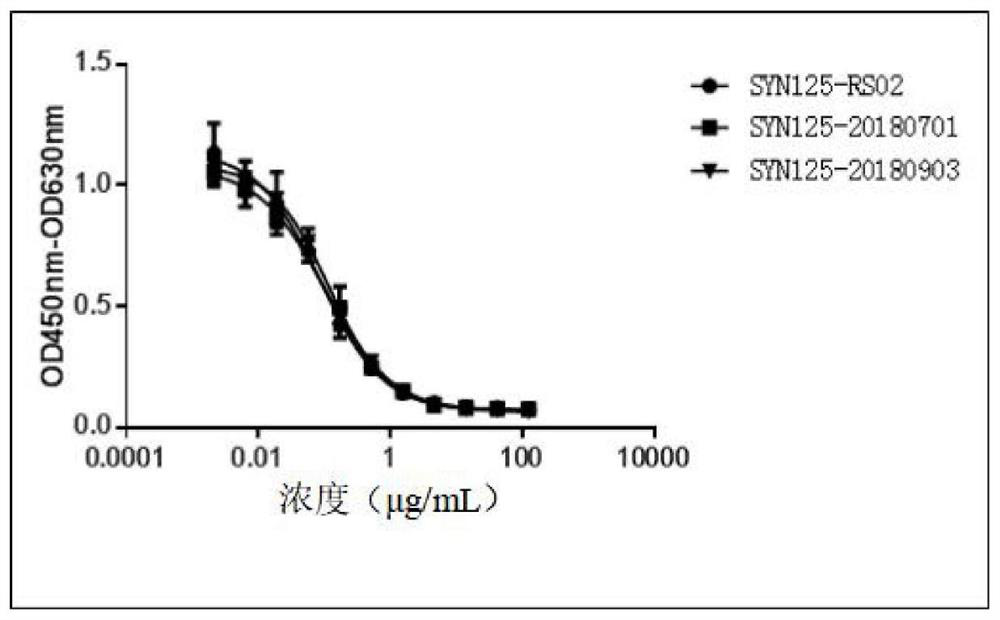
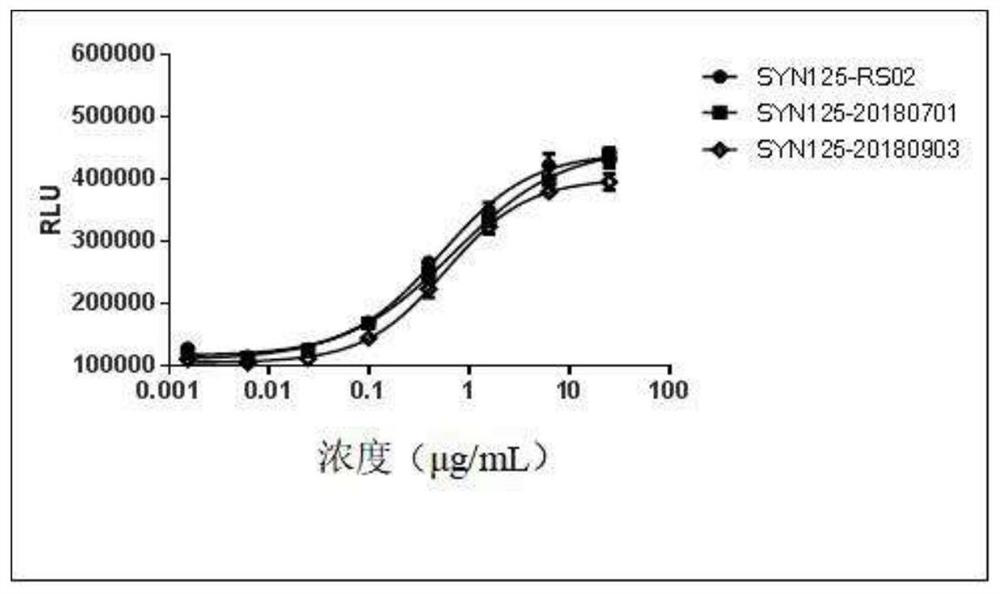
A stable anti-PD-1 antibody preparation and its use
A PD-1 and antibody technology, which is applied in the direction of antibodies, medical preparations with non-active ingredients, specific peptides, etc., can solve the problem of affecting the quality of anti-PD-1 antibody preparations, poor stability and protection of anti-PD-1 antibodies, and There are no obvious benefits to protect antibodies, etc., to achieve the effect of reducing the rate of chemical degradation reaction, improving chemical stability, and improving the comfort of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Antibody Preparation Formula 1
[0045] The content of each component in the preparation is as shown in Table 1:
[0046] Table 1 Antibody preparations
[0047] Element content Anti-PD-1 antibody SYN125 5mg / mL Citrate 20mM Sodium chloride 100mM Polysorbate-80 0.01% (w / v) pH 6.0
Embodiment 2
[0048] Embodiment 2 Antibody preparation formula 2
[0049] The content of each component in the preparation is as shown in Table 2:
[0050] Table 2 Antibody preparations
[0051]
[0052]
Embodiment 3
[0053] Example 3 Antibody preparation formula 3
[0054] The content of each component in the preparation is as shown in Table 1:
[0055] Table 3 Antibody Preparations
[0056] Element content Anti-PD-1 antibody SYN125 45mg / mL Citrate 20mM Sodium chloride 100mM Polysorbate-80 0.01% (w / v) pH 6.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com