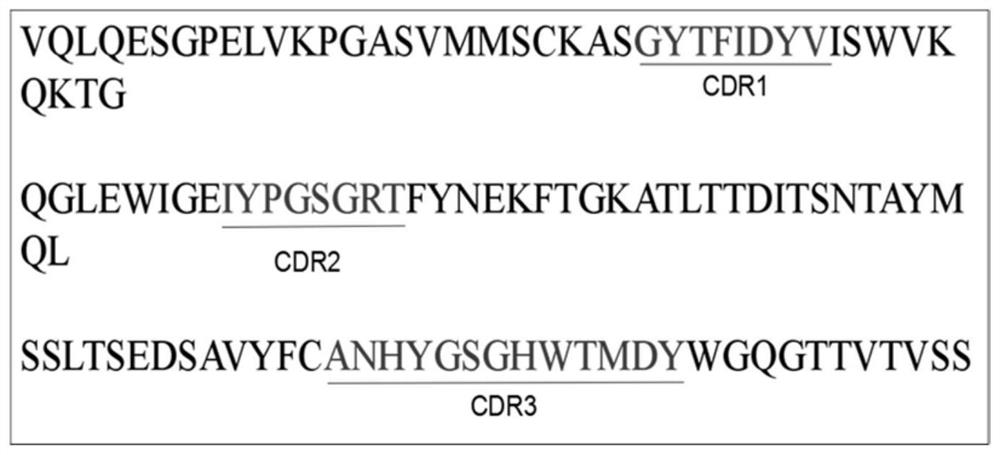
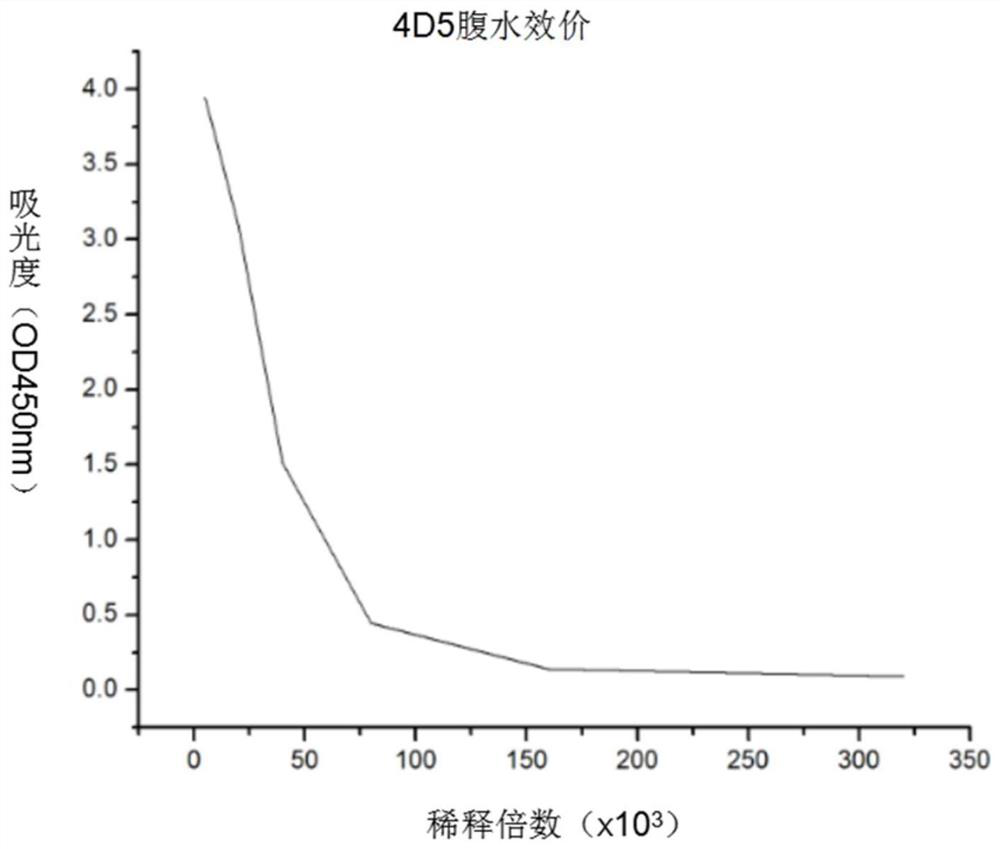
A kind of PRRS virus murine monoclonal antibody and its preparation method and application
A monoclonal antibody and blue ear virus technology, applied in the biological field, can solve problems such as inability to achieve large-scale on-site detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Antigen Preparation
[0061] Inactivated highly pathogenic PRRSV TP strain (that is, HP-PRRSV TP strain, obtained from reference: Yang, H., et al., CD163 knockout pigs are fully resistant to highly pathogenic porcine reproductive and respiratory syndrome virus.Antiviral Res , 2018.151: p.63-70.).
Embodiment 2
[0062] The preparation of embodiment 2 monoclonal antibody
[0063] 1. The preparation method of PRRS mouse-derived monoclonal antibody is as follows:
[0064] Animal immunization: 10-week-old female Balb / c mice (purchased from the Animal Center of Southern Medical University, Guangdong Province) were selected.
[0065] (1) Initial immunization: 300 μg of antigen (prepared in Example 1) and complete Freund's adjuvant were emulsified at a ratio of 1:1 by volume and injected subcutaneously at multiple points;
[0066] (2) Secondary immunization: 2 weeks later, the same amount of antigen as the first immunization plus Freund's incomplete adjuvant was emulsified at a ratio of 1:1 by volume and injected subcutaneously at multiple points;
[0067] (3) Three times of immunization: 2 weeks later, the same amount of antigen as the first immunization plus Freund's incomplete adjuvant was injected subcutaneously at multiple points (blood was collected 10 days later to measure its potenc...
Embodiment 3
[0089] Embodiment 3 Monoclonal antibody of the present invention distinguishes highly pathogenic blue ear virus and traditional blue ear virus
[0090] 1. ELISA experiment verified monoclonal antibody to distinguish highly pathogenic PRRS virus from traditional PRRS virus
[0091] (1) coating: dilute respectively PRRSV TP strain (the highly pathogenic PRRSV TP strain inactivated in embodiment 1, be called for short PRRSV TP strain) with carbonate coating buffer, PRRSV CH-1a strain (purchased from Shandong Lvdu Biotechnology Co., Ltd., referred to as PRRS CH-1A strain, PRRS JN strain, PRRS QT strain, PRRS LQ strain, PRRS TY strain (the virus strains were all purchased in Shandong Ludu Biotechnology Co., Ltd.), MARC-145 (Marc145 cells, purchased from Guangzhou Alexander Biotechnology Co., Ltd.). Add 0.1ml of coating solution containing PRRSV TP strain to the reaction wells of different polystyrene plates and the coating solution containing PRRSVCH-1a strain overnight at 4 ° C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com